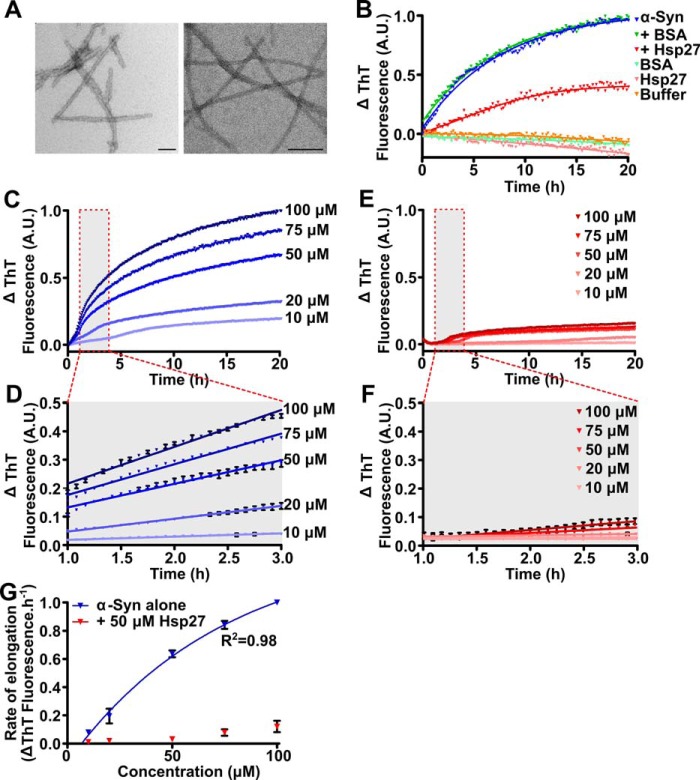
Figure 1.
Hsp27 inhibits the elongation of α-syn fibrils. A, amyloid seeds (left) and mature fibrils (right) of α-syn were imaged via transmission electron microscopy. Scale bars represent 100 nm. B, recombinant monomeric α-syn (50 μm) was incubated in 50 mm phosphate buffer (pH 7.4) in the absence or presence of 50 μm Hsp27 or the control protein BSA. After equilibration at 37 °C, α-syn seeds (2.5 μm monomer equivalent) were added to each sample containing monomeric α-syn and the elongation of these seeds was monitored via the change in ThT fluorescence at 490 nm over time. C–F, monomeric α-syn was incubated in 50 mm phosphate buffer (pH 7.4) at concentrations ranging from 10 to 100 μm in the absence (C) or presence (E) of 50 μm Hsp27. After equilibration at 37 °C, α-syn seeds (2.5 μm) were added to each sample and the elongation monitored via the change in ThT fluorescence at 490 nm over time. D and F, the data from 1–3 h (outlined in the red box in C and E) of incubation were fitted to a linear regression function. Data shown are mean ± S.E. of triplicate samples. G, the rate of elongation of the α-syn seeds, in the absence and presence of Hsp27, was calculated using values from these fits and correlated with the monomeric α-syn concentration. Data for α-syn alone were fitted to a Michaelis-Menten kinetic model. The data in C–F are representative of four independent repeats. The data in G are presented as the mean ± S.E. of these four repeats.