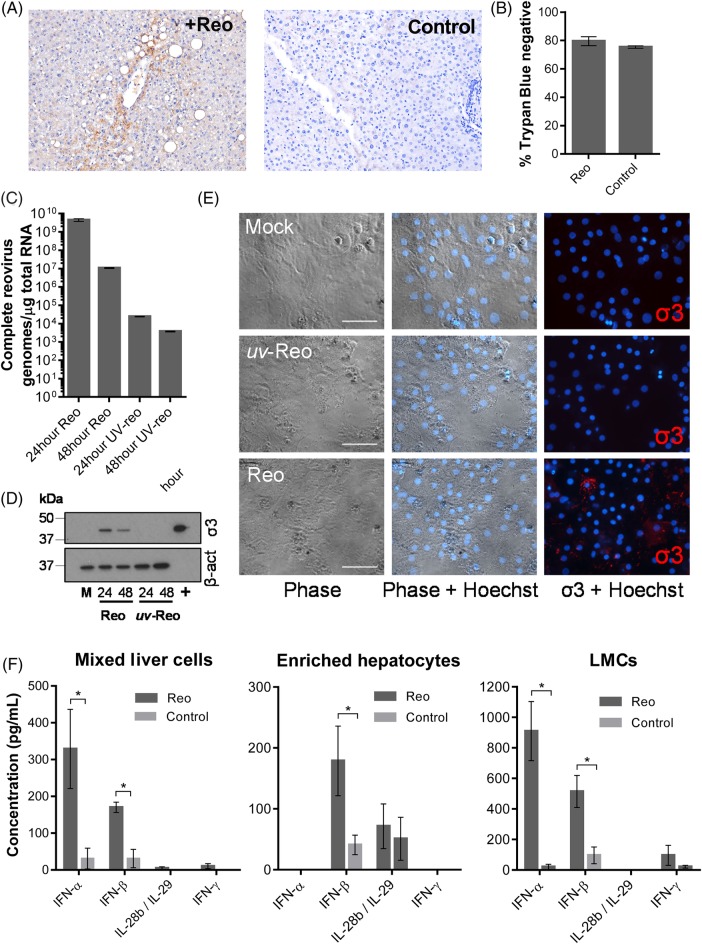
Figure 1.
Reo reaches normal liver tissue following intravenous injection and stimulates interferon (IFN) secretion from ex vivo liver cells. (A) Immunohistochemistry (IHC) for Reo σ3 capsid protein (brown) from normal liver tissue derived from a patient treated intravenously with Reo (left) or an untreated control (right). Slides are representative of the clinical trial series or six untreated controls. (B) Viability assay for enriched ex vivo hepatocytes treated using Phosphate Buffered Saline (PBS) or 10 PFU/cell Reo for 72 hours and assayed for membrane integrity by Trypan Blue staining. (C) quantitative reverse transcriptase (qRT)-PCR for Reo σ3. Primary human hepatocytes were treated using 1 PFU/cell Reo or uv-Reo and incubated for 24 or 48 hours prior to RNA extraction. (D) Western blot for Reo σ3 and β-actin. Primary human hepatocytes were treated using 1 PFU/cell Reo or uv-Reo and incubated for 24 or 48 hours. Mock (M)-treated hepatocytes and RNA purified from Reolysin stocks (+) served as negative and positive controls. (E) Human hepatocytes were infected with Reo, or uv-Reo as above, and subjected to immunofluorescence analysis for Reo σ3 capsid protein, with Hoechst nuclear counterstain. (F) ELISA for IFN-α, IFN-β, interleukin (IL)-28b/IL-29 and IFN-γ derived from ex vivo mixed liver cells, enriched hepatocytes and liver mononuclear cells (LMCs) following stimulation with Reo or PBS control. * signifies p<0.005.