Abstract
Multiple sclerosis (MS) is an autoimmune disease that affects the brain and spinal cord. The inflammasome is a multiprotein complex that contributes to the innate immune response in animal models of MS as well as in patients with the disease. Important to the care of patients with MS is the need for biomarkers that can predict disease onset, disease exacerbation, as well as response to treatment. In this study, we analyzed serum samples from 32 patients with MS and 120 age-matched controls, and provide receiver operator characteristic (ROC) curves with associated confidence intervals following analyses of serum samples from patients with MS, most of which had the relapsing-remitting form of the disease, and from healthy unaffected donors, and determine the sensitivity and specificity of inflammasome proteins as biomarkers of MS. We report that caspase-1 (1.662 ± 0.6024 difference between means), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (407.5 ± 35.79), and interleukin (IL)-18 (78.53 + 17.86) were elevated in the serum of MS patients when compared to controls. Interestingly, the levels of IL-1β (−0.5961 ± 0.265) were lower in the MS cohort. Importantly, the area under the curve (AUC) for ASC and caspase-1 were 0.9448 and 0.848, respectively. Taken together, these data suggest that ASC and caspase-1 could be potential candidate biomarkers for MS onset.
Keywords: inflammasome, biomarkers, serum, caspase-1, apoptosis-associated speck-like protein containing a caspase recruitment domain, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a progressive autoimmune disorder that affects the central nervous system (CNS). Pathologically, it is characterized by demyelination in the spinal cord and brain as well as the presence of inflammatory lesions (1). The current belief is that MS is an autoimmune disease characterized by autoreactive T lymphocytes that originate in the peripheral immune system and migrate to the CNS (2). Clinically, patients with MS present blurred vision, muscle weakness, fatigue, dizziness, as well as balance, and gait problems (1). Importantly, current therapies for MS target the inflammatory response, thus highlighting the relevance of further investigation on the immune response in MS (3–5).
In the United States, alone, there are 400,000 patients with MS and about 2 million patients worldwide (1). An important area of research in the field of MS is the identification of suitable biomarkers to predict who is at risk of developing MS, biomarkers of disease progression or exacerbation, as well as biomarkers of treatment response and prognosis (1).
The inflammasome is a key mediator of the innate immune response that in the CNS was first described to mediate inflammation after spinal cord injury (6). The inflammasome is a multiprotein complex involved in the activation of caspase-1 and the processing of the pro-inflammatory cytokines, interleukin (IL)-1β and IL-18 (7). The inflammasome contributes to the inflammatory response in MS. For instance, caspase-1 and IL-1 are present in MS plaques, and these proteins are also elevated in peripheral blood mononuclear cells of MS patients (8, 9). In addition, the NOD-like receptor protein-3 (NLRP3) inflammasome has been shown to play a role in the development of experimental autoimmune encephalomyelitis (EAE) in mice. Accordingly, mice deficient in NLRP3 were protected from developing EAE, while apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase-1-deficient mice also contributed to the disease (10–12).
In this study, we provide receiver operator characteristic (ROC) curves with associated confidence intervals following analyses of serum samples from patients with MS and from healthy unaffected donors. In addition, we determine the sensitivity and specificity of inflammasome proteins to examine the potential of inflammasome signaling proteins as biomarkers of MS.
Materials and Methods
Participants
Subjects were enrolled in the study Prospective Collection of Samples for Research according to IRB # 201301461 approved by Schulman Associates IRB for BioreclamationIVT. In this study, we analyzed serum samples from 120 normal donors and 32 patients who were diagnosed with MS. Samples were purchased from BioreclamationIVT. The normal donor group consisted of samples obtained from 60 male and 60 female donors in the age range of 20–70 years old. These patients had no clinical diagnosis of MS or any other disease at the time of blood donation. The age range in the MS group consisted of samples obtained from patients in the age range of 24–64 years old. As shown in Table 1, 20 patients were being treated with Tysabri, 20 were diagnosed with mild to moderate relapsing remitting MS (RRMS), 2 with secondary progressive MS (SPMS) (moderate), and 10 were unspecified. Three patients were treated with Tecfidera, one with Copaxone, two with Gilenya, one with Rebif, one with Betaseron, one with Ampyra, and three untreated with a medication for MS.
Table 1.
Subjects with multiple sclerosis (MS) gender.
| Gender | Age | Diagnosis | Medications | Race | Ethnicity | Condition |
|---|---|---|---|---|---|---|
| Female | 64 | MS | Ampyra 10 mg, Losartan 50 mg, Lyrica 50 mg, Tysabri 300 mg, Amlodipine 5 mg | Caucasian | RRMS—mild | |
| Male | 50 | Migraine, hypertension (HTN), MS, osteoarthritis (OA) | Baclofen 40 mg, Neurontin, Lopressor 0.25 mg, Omeprazole 40 mg, Effexor 37.5 mg, Tysabri 300 mg, Dulera 6 mg, Tizanidine 4 mg, Percocet 7.5–325 mg, Amitriptyline | Caucasian | ||
| Female | 46 | MS | Tysabri 300 mg, Abilify 5 mg, Ditropan 15 mg, Effexor 150 mg, Feosol 325 mg, Ketoconazole, Klonopin 2 mg, Topamax 200 mg, Wellbutrin 300 mg, Vicodin 7.5–750 mg | Caucasian | RRMS—mild | |
| Male | 47 | MS, OA, asthma | Tysabri 300 mg, Gabapentin 800 mg, Diazepam 5 mg, Vitamin D 50000 iu | Caucasian | RRMS—moderate | |
| Female | 48 | MS, osteoporosis, iron anemia | Provigil 200 mg, Escitalopram 20 mg, Tysabri 300 mg, Klonopin 0.5 mg, Fiorinal-Codeine 30–50 mg | Caucasian | Secondary progressive MS (SPMS)—moderate | |
| Female | 55 | MS, hypertension, depression | Effexor 200, Tysabri 300 mg, Abilify 2 mg, Cymbalta 60 mg, Modafinil 40 mg, Losartan 100 mg, Tramadol 50, Prilosec 20 mg | Caucasian | RRMS—mild | |
| Female | 47 | Peanut allergy, hypercholesterolemia, hypertension, osteopenia, vitamin D deficiency, relapse remitting multiple sclerosis (RRMS), seafood allergy | Losartan 100 mg, Hydrochlorothiazide 25 mg, Amlodipine 5 mg, Epipen 0.3 mg, Atorvastatin 20 mg, Benadryl 50 mg, Ibaridronate 0.5 mg, Trivora | Caucasian | ||
| Male | 30 | MS | Tecfidera 240 mg | Caucasian | Native American | RRMS—mild |
| Female | 34 | MS | Tysabri 300 mg | Caucasian | RRMS—Mild | |
| Female | 49 | MS, depression | Adderall 18 mg, Amlodipine 5 mg, Detrol, Hydrochlorothiazide 25 mg, Wellbutrin 300 mg, Ocuvite, Rebif 44 mcg, Topamax 50 mg, Vitamin D3 | Caucasian | RRMS—mild | |
| Female | 24 | MS, hypercholesterolemia, polycystic ovary syndrome (PCOS) | Neurontin 300 mg, Percocet 5–325mg, Tizanidine 2 mg, Tysabri 300 mg, Xanax 0.5 mg, Zoloft 50 mg | Caucasian | RRMS—moderate | |
| Male | 49 | MS, anxiety, depression | Clonazepam 0.5, Klonopin 0.5, Multivitamin, Neurontin 300, Wellbutrin 300, Provigil 100, Requip 0.5, Tysabri 300 mg/15 ml, Cyanocobalamin, Zoloft 100 mg | Caucasian | RRMS—moderate | |
| Female | 50 | MS | Cymbalta 60 mg, Ditropan XL 10 mg, Klonopin 0.5 mg, Lexapro 20 mg, Gabapentin 500 mg, Simvastatin 10 mg, Tysabri 300 mg, Wellbutrin 150 mg | Caucasian | RRMS—Mild | |
| Male | 38 | MS, eczema | Zyrtec, Advil, Tysabri | Caucasian | RRMS—mild | |
| Female | 62 | MS | Tysabri, Baclofen, Ampyra, Vitamin D, Aspirin 81 mg, Mutlivitamin, Cranberry, Statin | Caucasian | RRMS—mild | |
| Male | 51 | MS, hypoglycemia, asthma (unspecified) | Baclofen 10 mg, Ditropan 15 mg, Provigil 200 mg, Wellbutrin 150 mg, Multivitamin, Tysabri 300 mg | Caucasian | MS—moderate (Relapsing Remitting) | |
| Female | 38 | Migraine, MS | Synthroid 375 mg, Cytomel 50 mg, Vitamins, Copaxone 40 mg | Caucasian | ||
| Male | 32 | Migraine, MS | Gilenya 0.5 mg, Baclofen 20 mg, Adderall 10 mg, Klonopin 1 mg, Lyrica 75 mg, Venlafaxine 37.5 mg, Cambia 50 mg | Unknown | ||
| Female | 30 | Migraine, MS | Tysabri 300 mg, Adderall 20 mg, Topamax 50 mg, Ambien 10 mg | Caucasian | ||
| Male | 36 | Migraine, hypercholesterolemia, MS | Tysabri, Wellbutrin 50 mg, Lipitor 20 mg, Vitamin D3 | Caucasian | ||
| Female | 48 | MS, anxiety, depression, migraine | Topamax 25 mg, Keflex 250 mg, Cephalosporin 200 mg, Linzess 145mcg, Lexapro 10 mg, Flexeril 10 mg, Gilenya 0.5 mg | Caucasian | RRMS—mild | |
| Female | 55 | Migraine, hypercholesterolemia, MS, asthma | Betaseron 0.3 mg, Fluoxetine 20 mg, Atorvastatin 20 mg, Multivitamin, Calcium, Vitamin D, Aspirin, Probiotic, Maxalt | Caucasian | ||
| Female | 41 | Migraine, asthma, endometriosis, colitis, MS | Topamax 100 mg, Trexamet, Amitriptyline 25 mg, Clarinex, Zyrtec, Aspirin 81 mg | Caucasian | Native American | |
| Male | 37 | Migraine, MS, vertigo | Tysabri, Tyzanidine | Caucasian | ||
| Female | 41 | MS | Vitamin D 2,000 U, Trazodone 50 mg, Maxalt 10 mg, Valacyclovir 1, Medrol 4 mg, Alaprazolam 0.5 mg, Lyrica 50 mg, Provigil 200 mg, Cymbalta 60 mg, Tysabri 300 mg | Caucasian | RRMS—moderate | |
| Male | 43 | MS | Tysabri 300 mg | African | African-American | RRMS—mild |
| Female | 47 | Migraines, MS, psoriasis, infertility | B12, Tysabri 300mg/15ml, Xanax 0.5 mg, Advil | Caucasian | ||
| Female | 53 | Migraines, MS | Tecfidera 240 mg, Ampyra 10 mg, Aranesp, Topamax 100 mg, Myrbetriq 5 mg, Linzess, Sumatriptan, Treximet 85 mg, Ritalin 10 mg | Caucasian | ||
| Female | 54 | MS | Bystolic 2.5 mg, Sertraline 50 mg, Toviaz 4 mg, Tysabri 300 mg | Caucasian | RRMS—moderate | |
| Female | 47 | MS | Ambien 10 mg, Clorazepate 7.5 mg, Zoloft 50 mg | Caucasian | RRMS—mild | |
| Female | 52 | MS | Tecfidera 240 mg, Percocet 10–325 mg, Xanax 0.5 mg, Ambien 12.5 mg, Prevacid, Calcium 1,000 mg, Prolera, Cran Tab | Caucasian | RRMS—mild | |
| Female | 54 | MS | Baclofen 10 mg, Sarella 50 mg, Ampyra 10 mg, Lipitor 40 mg, Crestor 20 mg, Vesicare 10 mg, Synthroid 0.5 mg | Caucasian | SPMS—moderate | |
Simple Plex Assay
Concentration of inflammasome proteins caspase-1, ASC, IL-1β, and IL-18 in serum was analyzed as described in Ref. (13) using the Ella System (Protein System). The Simple Plex assay was analyzed by the Simple Plex Explorer software. Results shown correspond to the mean of each sample run in triplicates. The limit of quantitation for ASC was 13.1 pg/ml (lower limit of quantitation, LLOQ) and 8,000 pg/ml (upper limit of quantitation), for caspase-1, 0.66–1,000 pg/ml, for IL-1β, 0.21–840 pg/ml, and for IL-18, 0.390–3,660 pg/ml. The intra-assay mean for ASC was 47 ± 2.8 pg/ml (low QC ± SD) and 2,806 ± 152 pg/ml (high QC ± SD), for caspase-1, 5.3 ± 0.266 and 266 ± 17.8 pg/ml, for IL-1β, 8.66 ± 0.427 and 446 ± 16.5 pg/ml, and for IL-18,10.5 ± 0.524 and 569 ± 18.7 pg/ml. The inter-assay mean for ASC was 51.1 ± 5.5 and 2,551 ± 257 pg/ml, for caspase-1, 5.81 ± 0.816 and 269 ± 29 pg/ml, for IL-1β, 8.42 ± 0.342 and 410 ± 23.3 pg/ml, and for IL-18, 10.3 ± 0.882 and 587 ± 58.4 pg/ml.
Biomarker Analyses
Prism 7 software (GraphPad) was used to analyze the data obtained from the Simple Plex Explorer Software. Comparisons between groups were carried after identifying outliers followed by determination of the area under the ROC curve, as well as the 95% confidence interval (CI). The p-value of significance used was <0.05. Sensitivity and specificity of each biomarker was obtained for a range of different cut-off points. Samples that yielded a protein value below the level of detection of the assay were not included in the analyses for that particular analyte.
Results
Caspase-1, ASC, and IL-18 Are Elevated in the Serum of MS Patients
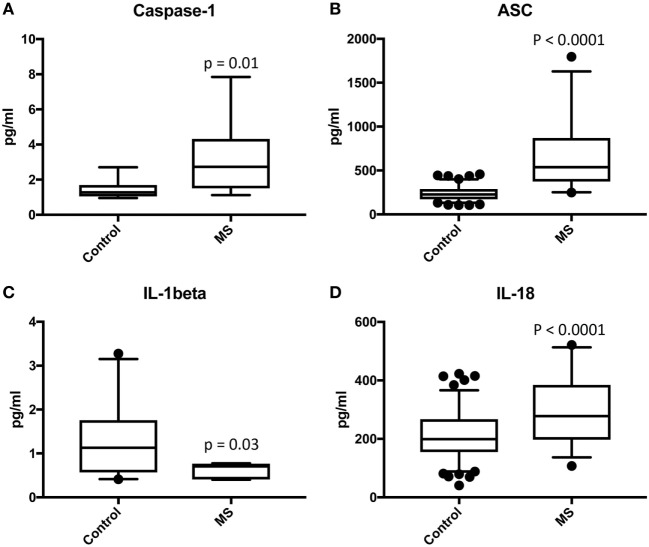
We analyzed serum samples from MS patients and compared them to serum from healthy/control individuals using a Simple Plex assay (Protein Simple) for the protein expression of the inflammasome signaling proteins caspase-1, ASC, IL-1β, and IL-18 (Figure 1). Accordingly, we found that the protein levels of caspase-1, ASC, and IL-18 in the serum of MS patients was higher than in the control group. However, the levels of IL-1β were lower in the MS than controls. These findings are consistent with previous reports indicating a role for the inflammasome in the pathology of MS (10, 11, 14).
Figure 1.
Inflammasome proteins are elevated in the serum of MS patients. Protein levels in pg/ml of caspase-1 (A), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (B), IL-1β (C), and IL-18 (D) in serum samples from patients with MS and healthy donors. p-value of significance is shown above each box plot. Box and whiskers are shown for the 5th and 95th percentile. Caspase-1: N = 9 control and 19 MS; ASC: N = 115 control and 32 MS; IL-1β: N = 21 control and 8 MS; and IL-18: N = 119 control and 32 MS.
ASC and Caspase-1 Are Promising Serum Biomarkers of MS
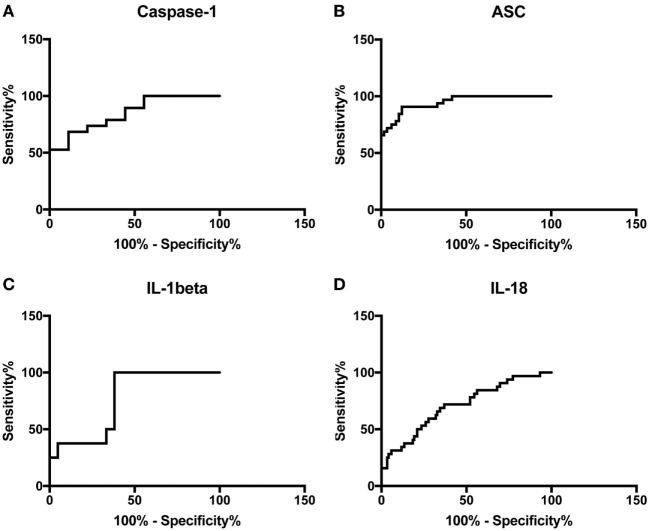
To then determine if these inflammasome signaling proteins have the potential to be reliable biomarkers for MS pathology, we determined the area under the curve (AUC) for caspase-1 (Figure 2A), ASC (Figure 2B), IL-1β (Figure 2C), and IL-18 (Figure 2D). Of the proteins that we measured, ASC was shown to be the best potential biomarker (Figure 3) with an AUC of 0.9448 and a CI between 0.9032 and 0.9864 (Table 2). In addition, caspase-1 with an AUC of 0.848 and a CI between 0.703 and 0.9929 is also promising biomarker of MS.
Figure 2.
Receiver operator characteristic curves for caspase-1 (A), apoptosis-associated speck-like protein containing a caspase recruitment domain (B), IL-1β (C), and IL-18 (D) from serum samples of multiple sclerosis and healthy donors.
Figure 3.
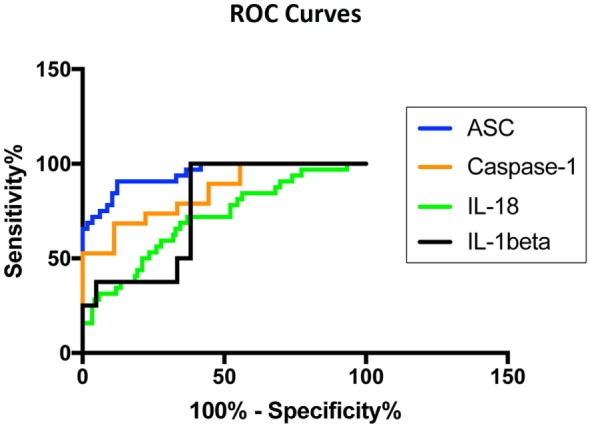
Inflammasome proteins in serum as biomarkers of multiple sclerosis (MS). Receiver operator characteristic curves for caspase-1 (orange), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (blue), IL-1β (black), and IL-18 (green). Caspase-1: N = 9 control and 19 MS; ASC: N = 115 control and 32 MS; IL-1β: N = 21 control and 8 MS; and IL-18: N = 119 control and 32 MS.
Table 2.
Receiver operator characteristic analysis results for inflammasome signaling proteins in serum and cut-off point analyses for inflammasome signaling proteins in serum.
| Biomarker | Area | SE | 95% CI | P value | |
|---|---|---|---|---|---|
| Caspase-1 | 0.848 | 0.07394 | 0.703–0.9929 | 0.0034 | |
| Apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) | 0.9448 | 0.02122 | 0.9032–0.9864 | <0.0001 | |
| IL-1 beta | 0.7619 | 0.0925 | 0.5806–0.9432 | 0.0318 | |
| IL-18 | 0.7075 | 0.05216 | 0.6052–0.8097 | 0.0003 | |
| Biomarker | Cut-off point (pg/ml) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| Caspase-1 | >1.302 | 89 | 56 | 81 | 71 |
| ASC | >352.4 | 84 | 90 | 70 | 95 |
| IL-1 beta | <0.825 | 100 | 62 | 50 | 100 |
| IL-18 | >190.1 | 84 | 44 | 29 | 91 |
Furthermore, the cut-off point for ASC was 352.4 pg/ml with 84% sensitivity and 90% specificity (Table 2). For caspase-1, the cut-off point was 1.302 pg/ml with 89% sensitivity and 56% specificity (Table 2). Moreover, we found that with regards to ASC for a 100% sensitivity, the cut-off point was 247.2 pg/ml with 58.26% specificity, and for 100% specificity, the cut-off point was 465.1 pg/ml with 65.63% sensitivity. In the case of caspase-1, for 100% sensitivity, the cut-off point was 1.111 pg/ml with 44.44% specificity. For 100% specificity, the cut-off point was 2.718 pg/ml with 52.63% sensitivity. Thus, these findings indicate that caspase-1 and ASC are promising biomarkers for MS.
ASC Is a Promising Biomarker of MS Severity in Serum
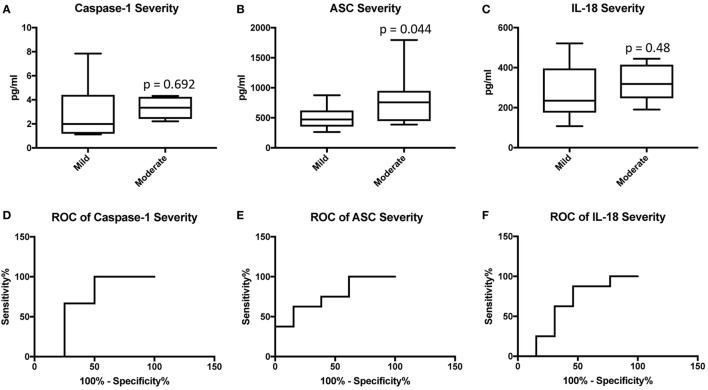
We then separated the groups into mild and moderate MS. Accordingly, we found that the protein level of ASC was higher in the serum of MS patients with moderate disease onset than in the mild group (p = 0.044) (Figure 4B), whereas the caspase-1 (Figure 4A) and IL-18 (Figure 4C) levels were not statistically different between the two groups. To then determine if inflammasome proteins can be used as diagnostic biomarkers of disease severity, we determined the AUC for caspase-1 (Figure 4D), ASC (Figure 4E), and IL-18 (Figure 4F). Of the proteins that we measured, ASC was shown to be the best biomarker with an AUC of 0.7596 and a CI between 0.5437 and 0.9756 (Table 3). Furthermore, the cut-off point for ASC was 537.5 pg/ml with 75% sensitivity and 62% specificity (Table 3). Thus, these findings indicate that ASC is a promising diagnostic biomarker for MS severity.
Figure 4.
Inflammasome proteins in serum as biomarkers of multiple sclerosis (MS) severity. Protein levels in pg/ml of caspase-1 (A), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (B), and IL-18 (C) in serum samples from patients with MS and healthy donors. p-value of significance is shown above each box plot. Box and whiskers are shown for the fifth and 95th percentile. Receiver operator characteristic curves for caspase-1 severity (mild vs. moderate) (D), ASC (E), and IL-18 (F). Caspase-1: N = 8 mild and 6 moderate; ASC: N = 13 mild and 8 moderate; and IL-18: N = 13 mild and 8 moderate.
Table 3.
Receiver operator characteristic analysis results for inflammasome signaling proteins in serum and cut-off point analyses for inflammasome signaling proteins in serum as markers of multiple sclerosis severity (mild vs. moderate).
| Biomarker | Area | SE | 95% CI | P value | |
|---|---|---|---|---|---|
| Caspase-1 | 0.6667 | 0.155 | 0.3629–0.9704 | 0.30 | |
| ASC | 0.7596 | 0.11 | 0.5437–0.9756 | 0.05 | |
| IL-18 | 0.6346 | 0.12 | 0.3925–0.8767 | 0.31 | |
| Biomarker | Cut-off point (pg/ml) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| Caspase-1 | >1.776 | 100 | 50 | 73 | 100 |
| ASC | >537.5 | 75 | 62 | 76 | 60 |
| IL-18 | >238.2 | 88 | 54 | 75 | 73 |
Discussion
Multiple sclerosis is an autoimmune disease that affects the brain and spinal cord characterized by the presence of inflammatory lesions and demyelination (1). In MS, T-cells originating in the peripheral immune response and eventually infiltrate the CNS, producing demyelination and axon degeneration (15). The source of autoimmunity in MS remains to be identified. However, anti-inflammatory therapies have been shown to be efficient in reducing disease relapse and disease progression (3–5). It is possible that in addition to infiltrating T-cells that attack the myelin, infections that target the brain may activate an inflammatory response that then goes on to attack oligodendrocytes (16). In support of the role of inflammation on MS, IL-17 has been found to play an important role in the pathology of MS. IL-17 has been found in brain lesions of patients with MS as well as in peripheral blood mononuclear cells from these patients (17, 18). As a result, studies are being conducted to target IL-17 with neutralizing antibodies (19).
Multiple sclerosis remains the most common neurological disability in young adults (20). The pathological manifestations of MS include inflammation, demyelination-neurodegeneration, axonal damage, remyelination-repair, and gliosis (20). A better understanding of the molecular mechanisms contributing to these factors represents an opportunity to develop personalized therapies. From the understanding of these molecular mechanisms, one can identify potential biomarkers that can be used to guide the diagnosis, prognosis, and treatment of MS patients.
Importantly, many publications on the field of biomarker research lack adequate measures to determine the likelihood of elevated proteins in diseased cohorts as biomarkers of a particular disease. AUC values are important in biomarker analysis for this value does not change with the prevalence of a given outcome within the cohort used for that study. Moreover, it plots the sensitivity and specificity across the range of biomarker concentrations (21). In this study, in accordance with standards of biomarker analyses, we provide AUC values with confidence intervals, sensitivities, and specificities to identify the inflammasome proteins caspase-1, ASC, IL-1β, and IL-18 as suitable biomarkers that can be used in the care of patients with MS. A perfect AUC value is 1.0, where 100% of subjects in the population will be correctly classified as having MS or not. In contrast, an AUC of 0.5 signifies that subjects are randomly classified as either positive or negative for MS, which has no clinical utility. It has been suggested that an AUC between 0.9 and 1.0 applies to an excellent biomarker; from 0.8 to 0.9, good; 0.7 to 0.8, fair; 0.6 to 0.7, poor; and 0.5 to 0.6, fail (21).
The initial manifestation of demyelination is referred to as clinically isolated syndrome (CIS). Following CIS is the clinical manifestation of MS, which is referred to as clinically definite MS (CDMS) (22). The International Advisory Committee on Clinical Trials in MS describes MS with terms, such as progressing, not progressing, active, or not active as well as RRMS, and progressive MS. Progressive MS is then subdivided into primary progressive MS (PPMS) or SPMS (23). Patients with RRMS present new or recurrent symptoms with lack of disease progression between relapses. Patients with PPMS have no prior history of RRMS and present a loss of neurological function with occasional temporary minor improvements. SPMS is used to denote patients with RRMS who are progressing (24). As a result, due to the different manifestations of the disease, there is a need to find objective biomarkers that can stratify the different clinical scenarios that MS patients present.
Pharmacogenomic biomarkers (DNA, RNA) offer great potential in the care of MS patients. Recently, several genes have been identified to be associated with the response to IFNβ treatment in MS patients, including genes encoding for type I IFN and IFN regulatory transcription factors (25, 26). In addition, other genes have been associated with a lack of response to IFNβ treatment (27). However, future studies are needed to corroborate these findings (28). As a result, to date, magnetic resonance imaging (MRI) remains the main tool used in the diagnosis and monitoring of treatment in this patient population. MRI can image the changes in the blood–brain barrier associated with inflammatory demyelination. Moreover, using the most recent criteria allows for accurate diagnosis of MS with just one scan (29). Similarly, MRI offers strong predictability on the effect of therapy on the rate of relapse (30).
The NLRP3 inflammasome plays a major role in the pathology of MS (12). Interestingly, IFN-β which is used as therapy in MS, acts as an inhibitor of NLRP3 inflammasome activation in the EAE model (31, 32). In addition, in the EAE model the pyrin inflammasome has been shown to be responsible for the production of IL-1β by hematopoietic cells following stimulation with pertussis toxin (14).
In humans, higher levels of IL-18 have been previously detected in the serum and cerebrospinal fluid of patients with MS (33, 34). In this study, we detected a statistically significant higher level of IL-18 in the serum of MS patients when compared to healthy subjects, consistent with a high AUC (0.7075).
In the EAE animal model of MS, IL-1β plays a major role in this demyelinating disease (35). However, in humans conflicting data exists regarding the levels of IL-1β in MS patients. Accordingly, some studies indicate that IL-1β is elevated in the serum and CSF of patients with MS. In this cohort, patients with RRMS were considered clinically active (36, 37); however, other studies failed to detect IL-1β in the body fluids of patients with MS (38, 39). In this study, the levels of IL-1β were significantly lower in the MS group than in the control group.
In humans, caspase-1 is present in MS plaques and is elevated in peripheral blood mononuclear cells (8, 40). In this study, we found higher protein levels of caspase-1 in the serum of MS patients consistent with a high AUC for caspase-1 (0.848). ASC is a promising therapeutic target for CNS inflammation (6, 7, 41, 42). In the EAE model of MS, ASC plays a critical role in disease exacerbation together with caspase-1 (43). In our analysis, ASC was the most promising biomarker with an AUC of 0.9448.
Thus, based on these findings caspase-1 and ASC are promising biomarker with a high AUC value and a high sensitivity. Importantly, we believe that a combination of caspase-1 and ASC as biomarkers for MS with other diagnostic criteria has the potential to increase the sensitivity of these biomarkers for MS beyond what we describe here. In addition, our findings suggest that ASC could potentially be used as a diagnostic biomarker of disease severity, since we found higher levels of ASC in the moderate MS group when compared to the mild MS group, in addition to an AUC of 0.7596.
Some clinically used biomarkers, such as serum aquaporin four antibodies (AQP4-IgG), which are used to differentiate between patients with MS and patients with neuromyelitis optica, have a median sensitivity of 62.3% with a range between 12.5 and 100%, depending on the assay used for the measurements (44).
Since the 1960s immunoglobulin (Ig) G oligoclonal bands (OCB) have been used as a classic biomarker in the diagnosis of MS (45). However, the specificity of IgG-OCB is only 61%, as a result, other diagnostic criteria is needed to clinically determine the diagnosis of MS (46), yet CSF-restricted IgG-OCB is a good predictor for conversion from CIS to CDMS, independently of MRI (47). Similar results have been obtained when analyzing IgM-OCB (48). Interestingly, IgG against measles, rubella, and varicella zoster (MRZ) are present in the CSF of MS patients, thus MRZ-specific IgG have the potential to be used as biomarkers of MS diagnosis (49). Similarly, when interpreting the results of this study, it is possible that the effects seen in these proteins is more related to an activation of the innate immune response rather than being MS-specific, especially for all other proteins, but ASC, which has a 90% specificity in this cohort of samples.
This study was carried in accordance with the standards for reporting diagnostic accuracy studies (STARD). Based on the STARD standards, the study presents certain limitations. For instance, serum used in this study was obtained from a bank of samples from MS patients. Thus, when samples were initially collected, the study of inflammasome signaling proteins was not envisioned. As a result, data collection was planned after collection of samples, and eligibility criteria did not consider the role of inflammation in this patient population. This has resulted in the diverse patient population used in this study. Therefore, a more controlled study-design is needed in the future to extend the results of the present study. In addition, most of the samples used in this study came from Caucasian patients. In future studies, we need to look at samples from other races, including Hispanics and patients of African descent. Moreover, some patients in this study were being treated for other conditions; as a result, it is possible that some of the effects on inflammasome protein expression in serum are not related to MS pathology, but to the other indications that patients presented, such as hypertension or hypercholesterolemia, among others.
It is important to highlight that samples used in this study came from patients that were treated for MS with drugs, such as Tysabri, which targets integrin receptors (50). The fact that we were able to detect higher levels of inflammasome proteins despite treatment, suggests that there is still a need for drugs to target the inflammasome and other components of the immune response as therapies for MS. Moreover, another limitation of this study is that only 3 of the 32 samples belong to patients that were not receiving any treatment for MS at the time of collection. Therefore, future studies should look at these proteins in serum samples from treatment-naïve MS patients.
In addition, future research is needed to better determine the prognostic potential of these inflammasome markers on the pathology of MS. In addition, more studies are needed looking at larger patient populations to better determine the cut-off points that will give the higher specificity and sensitivity to each biomarker as a diagnostic tool of MS disease. There is also a need to look at the different levels of inflammasome proteins in the different types of MS patients as well as in patients receiving different therapies. Importantly, in this study we have identified caspase-1 and ASC as potential biomarkers of MS pathology with high AUC values; 0.9448 and 0.848, respectively with sensitivities above 80% and in the case of ASC a specificity of 90%.
Ethics Statement
Subjects were enrolled in the study Prospective Collection of Samples for Research according to IRB # 201301461 approved by Schulman Associates IRB for BioreclamationIVT. Samples used in this study were purchased from BioreclamationIVT.
Author Contributions
JV contributed to the design of the experiments, he ran the experiments, performed data analyses, and wrote the manuscript. RK contributed to the design of the experiments, performed data analyses, and wrote the manuscript. WD contributed to the design of the experiments, performed data analyses, and wrote the manuscript.
Conflict of Interest Statement
JV, RK, and WD are co-founders and managing members of InflamaCORE, LLC, and have patents on inflammasome proteins as biomarkers of injury and disease as well as on targeting inflammasome proteins for therapeutic purposes.
Footnotes
Funding. This work was supported by a STTR grant (NS086274) from the NINDS/NIH to RK and WD.
References
- 1.Compston A. The pathogenesis and basis for treatment in multiple sclerosis. Clin Neurol Neurosurg (2004) 106:246–8. 10.1016/j.clineuro.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 2.Hohlfeld R, Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad Sci U S A (2004) 101(Suppl 2):14599–606. 10.1073/pnas.0404874101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cossburn MD, Harding K, Ingram G, El-Shanawany T, Heaps A, Pickersgill TP, et al. Clinical relevance of differential lymphocyte recovery after alemtuzumab therapy for multiple sclerosis. Neurology (2013) 80:55–61. 10.1212/WNL.0b013e31827b5927 [DOI] [PubMed] [Google Scholar]
- 4.Frau J, Coghe G, Fenu G, Lorefice L, Cocco E. Rescue therapy with alemtuzumab in multiple sclerosis post-natalizumab puerperium reactivation. Neurol Sci (2017) 39(2):389–90. 10.1007/s10072-017-3135-y [DOI] [PubMed] [Google Scholar]
- 5.Steinman L. Induction of new autoimmune diseases after alemtuzumab therapy for multiple sclerosis: learning from adversity. JAMA Neurol (2017) 74:907–8. 10.1001/jamaneurol.2017.0325 [DOI] [PubMed] [Google Scholar]
- 6.de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci (2008) 28:3404–14. 10.1523/JNEUROSCI.0157-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamczak SE, De Rivero Vaccari JP, Dale G, Brand FJ, III, Nonner D, Bullock MR, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab (2014) 34:621–9. 10.1038/jcbfm.2013.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ming X, Li W, Maeda Y, Blumberg B, Raval S, Cook SD, et al. Caspase-1 expression in multiple sclerosis plaques and cultured glial cells. J Neurol Sci (2002) 197:9–18. 10.1016/S0022-510X(02)00030-8 [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Goods BA, Raddassi K, Nepom GT, Kwok WW, Love JC, et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med (2015) 7:287ra274. 10.1126/scitranslmed.aaa8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlan R, Martino G, Galbiati F, Poliani PL, Smiroldo S, Bergami A, et al. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol (1999) 163:2403–9. [PubMed] [Google Scholar]
- 11.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol (2010) 185:974–81. 10.4049/jimmunol.0904145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue M, Williams KL, Gunn MD, Shinohara ML. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A (2012) 109:10480–5. 10.1073/pnas.1201836109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand FJ, III, Forouzandeh M, Kaur H, Travascio F, De Rivero Vaccari JP. Acidification changes affect the inflammasome in human nucleus pulposus cells. J Inflamm (Lond) (2016) 13:29. 10.1186/s12950-016-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumas A, Amiable N, De Rivero Vaccari JP, Chae JJ, Keane RW, Lacroix S, et al. The inflammasome pyrin contributes to pertussis toxin-induced IL-1beta synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS Pathog (2014) 10:e1004150. 10.1371/journal.ppat.1004150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol (2015) 15:545–58. 10.1038/nri3871 [DOI] [PubMed] [Google Scholar]
- 16.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci (2008) 31:247–69. 10.1146/annurev.neuro.30.051606.094313 [DOI] [PubMed] [Google Scholar]
- 17.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler (1999) 5:101–4. 10.1177/135245859900500206 [DOI] [PubMed] [Google Scholar]
- 18.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med (2002) 8:500–8. 10.1038/nm0502-500 [DOI] [PubMed] [Google Scholar]
- 19.Havrdova E, Belova A, Goloborodko A, Tisserant A, Wright A, Wallstroem E, et al. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J Neurol (2016) 263:1287–95. 10.1007/s00415-016-8128-x [DOI] [PubMed] [Google Scholar]
- 20.Katsavos S, Anagnostouli M. Biomarkers in multiple sclerosis: an up-to-date overview. Mult Scler Int (2013) 2013:340508. 10.1155/2013/340508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics (2013) 9:280–99. 10.1007/s11306-012-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, et al. Conversion from clinically isolated syndrome to multiple sclerosis: a large multicentre study. Mult Scler (2015) 21:1013–24. 10.1177/1352458514568827 [DOI] [PubMed] [Google Scholar]
- 23.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol (2014) 72(Suppl 1):1–5. 10.1159/000367614 [DOI] [PubMed] [Google Scholar]
- 24.Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev (2014) 13:518–24. 10.1016/j.autrev.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 25.Cunningham S, Graham C, Hutchinson M, Droogan A, O’rourke K, Patterson C, et al. Pharmacogenomics of responsiveness to interferon IFN-beta treatment in multiple sclerosis: a genetic screen of 100 type I interferon-inducible genes. Clin Pharmacol Ther (2005) 78:635–46. 10.1016/j.clpt.2005.08.018 [DOI] [PubMed] [Google Scholar]
- 26.Coyle PK. Pharmacogenetic biomarkers to predict treatment response in multiple sclerosis: current and future perspectives. Mult Scler Int (2017) 2017:6198530. 10.1155/2017/6198530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsareva E, Kulakova O, Boyko A, Favorova O. Pharmacogenetics of multiple sclerosis: personalized therapy with immunomodulatory drugs. Pharmacogenet Genomics (2016) 26:103–15. 10.1097/FPC.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 28.Martire S, Navone ND, Montarolo F, Perga S, Bertolotto A. A gene expression study denies the ability of 25 candidate biomarkers to predict the interferon-beta treatment response in multiple sclerosis patients. J Neuroimmunol (2016) 292:34–9. 10.1016/j.jneuroim.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 29.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol (2017) 17(2):162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 30.Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol (2013) 12:669–76. 10.1016/S1474-4422(13)70103-0 [DOI] [PubMed] [Google Scholar]
- 31.Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, et al. Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal (2012) 5:ra38. 10.1126/scisignal.2002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue M, Chen PH, Siecinski S, Li QJ, Liu C, Steinman L, et al. An interferon-beta-resistant and NLRP3 inflammasome-independent subtype of EAE with neuronal damage. Nat Neurosci (2016) 19:1599–609. 10.1038/nn.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losy J, Niezgoda A. IL-18 in patients with multiple sclerosis. Acta Neurol Scand (2001) 104:171–3. 10.1034/j.1600-0404.2001.00356.x [DOI] [PubMed] [Google Scholar]
- 34.Chen YC, Chen SD, Miao L, Liu ZG, Li W, Zhao ZX, et al. Serum levels of interleukin (IL)-18, IL-23 and IL-17 in Chinese patients with multiple sclerosis. J Neuroimmunol (2012) 243:56–60. 10.1016/j.jneuroim.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Levesque SA, Pare A, Mailhot B, Bellver-Landete V, Kebir H, Lecuyer MA, et al. Myeloid cell transmigration across the CNS vasculature triggers IL-1beta-driven neuroinflammation during autoimmune encephalomyelitis in mice. J Exp Med (2016) 213:929–49. 10.1084/jem.20151437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauser SL, Doolittle TH, Lincoln R, Brown RH, Dinarello CA. Cytokine accumulations in CSF of multiple sclerosis patients: frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology (1990) 40:1735–9. 10.1212/WNL.40.11.1735 [DOI] [PubMed] [Google Scholar]
- 37.Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N, et al. The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: the elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol (2009) 207:101–6. 10.1016/j.jneuroim.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 38.Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol (1991) 32:67–74. 10.1016/0165-5728(91)90073-G [DOI] [PubMed] [Google Scholar]
- 39.Tsukada N, Miyagi K, Matsuda M, Yanagisawa N, Yone K. Tumor necrosis factor and interleukin-1 in the CSF and sera of patients with multiple sclerosis. J Neurol Sci (1991) 104:230–4. 10.1016/0022-510X(91)90315-X [DOI] [PubMed] [Google Scholar]
- 40.Huang WX, Huang P, Hillert J. Increased expression of caspase-1 and interleukin-18 in peripheral blood mononuclear cells in patients with multiple sclerosis. Mult Scler (2004) 10:482–7. 10.1191/1352458504ms1071oa [DOI] [PubMed] [Google Scholar]
- 41.de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab (2009) 29:1251–61. 10.1038/jcbfm.2009.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Rivero Vaccari JP, Dietrich WD, Keane RW. Therapeutics targeting the inflammasome after central nervous system injury. Transl Res (2016) 167:35–45. 10.1016/j.trsl.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw PJ, Lukens JR, Burns S, Chi H, Mcgargill MA, Kanneganti TD. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol (2010) 184:4610–4. 10.4049/jimmunol.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarius S, Wildemann B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol (2013) 23:661–83. 10.1111/bpa.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stangel M, Fredrikson S, Meinl E, Petzold A, Stuve O, Tumani H. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol (2013) 9:267–76. 10.1038/nrneurol.2013.41 [DOI] [PubMed] [Google Scholar]
- 46.Teunissen CE, Malekzadeh A, Leurs C, Bridel C, Killestein J. Body fluid biomarkers for multiple sclerosis – the long road to clinical application. Nat Rev Neurol (2015) 11:585–96. 10.1038/nrneurol.2015.173 [DOI] [PubMed] [Google Scholar]
- 47.Tintore M, Rovira A, Rio J, Tur C, Pelayo R, Nos C, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology (2008) 70:1079–83. 10.1212/01.wnl.0000280576.73609.c6 [DOI] [PubMed] [Google Scholar]
- 48.Villar LM, Masjuan J, Gonzalez-Porque P, Plaza J, Sadaba MC, Roldan E, et al. Intrathecal IgM synthesis predicts the onset of new relapses and a worse disease course in MS. Neurology (2002) 59:555–9. 10.1212/WNL.59.4.555 [DOI] [PubMed] [Google Scholar]
- 49.Brettschneider J, Tumani H, Kiechle U, Muche R, Richards G, Lehmensiek V, et al. IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS One (2009) 4:e7638. 10.1371/journal.pone.0007638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selewski DT, Shah GV, Segal BM, Rajdev PA, Mukherji SK. Natalizumab (Tysabri). AJNR Am J Neuroradiol (2010) 31:1588–90. 10.3174/ajnr.A2226 [DOI] [PMC free article] [PubMed] [Google Scholar]