Abstract
Molecular imaging is critical to personalized and precision medicine. Although singly targeted imaging probes are making an impact both clinically and preclinically, molecular imaging strategies using bispecific probes have enabled improved visualization of cancer in recent years through synergistic targeting of two ligands. In this Focus on Molecular Imaging review, we outline how peptide-, antibody-, and nanoparticle-based platforms have affected this emerging strategy, providing examples and pointing out areas in which the greatest clinical impact may be realized.
Keywords: molecular imaging, dual-targeted, bispecific antibody fragment, cancer, positron emission tomography
As personalized and precision medicine have come to the forefront of cancer therapies, a need for targeted imaging agents has also arisen. Great advances have been made in molecular imaging using highly specific peptide-, antibody-, and nanoparticle-based agents, enabling noninvasive, longitudinal, in-depth information in preclinical cancer models, as well as clinically about a patient’s disease state. In some instances, however, greater imaging contrast and higher specificity for diseased tissues are still warranted. Dual-targeted molecular imaging agents have filled this vacancy, providing specific uptake in tissues of interest that is superior to their corresponding monomeric counterparts due to their increased maximum binding and improved pharmacokinetic profiles. While the affinity of each monomer is ideally maintained, specific interaction with two different targets allows these dual-targeted probes to display enhanced avidity. These agents may target two receptors/proteins on a cancer cell’s surface, the tumor microenvironment, or on immune cells.
In addition to molecular imaging, dual-targeting strategies have also been applied to generate therapeutic agents for disease treatment. More than 60 bispecific antibodies have been developed for potential therapeutic purposes (1). One of the major applications of bispecific antibodies is for redirecting immune effector cells to tumor cells. Bispecific T-cell engagers, binding to T cells (e.g., via the CD3 receptor) and tumor cells (via a tumor-specific target), direct T cells to tumor cells for cytotoxic activity; among them, blinatumomab was approved by the Food and Drug Administration in 2014 for acute B-cell lymphoblastic leukemia treatment (2). An additional therapeutic application of bispecific antibodies is for simultaneously blocking two different signaling pathways, a feat that could not be achieved with a monospecific antibody. One representative of this type of antibody is duligotuzumab, which targets both the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 3 (HER3), overcoming the drug resistance often encountered by patients receiving the anti-EGFR drug cetuximab (3).
Of note, bispecific platforms are being used in a unique manner for pretargeting strategies (4). Therein, an agent that is targeted to a cancer cell surface marker and also has specificity for a biorthogonal compound (such as a chelator) is injected and allowed to accumulate at a tumor site and clear elsewhere. After this, the second compound, with an imaging tag attached (such as a PET isotope), is injected and preferentially accumulates at the tumor site. Promising preclinical and initial clinical results have been reported, making this another area for bispecific tracers to make an impact in future cancer care.
Collectively, dual-targeted strategies have been demonstrated to be promising approaches for both imaging and therapy. Therapeutic applications of bispecific agents have been thoroughly outlined elsewhere (5); therefore, we herein aim to provide a brief overview of the uses and development of dual-targeted molecular imaging agents, based on peptide, antibody, and nanoparticle platforms (Table 1).
TABLE 1.
Representative Examples of Dual-Targeted Molecular Imaging Agents
| Agent | Imaging modality | Reference |
| Peptide-based | ||
| Targets | ||
| PSMA and GRPR | PET | 7 |
| GRPR and integrin αvβ3 | PET | 11–13 |
| uPAR and integrin αvβ3 | PET | 8 |
| Antibody- and antibody fragment–based | ||
| Targets | ||
| EGFR and HER2 | PET/SPECT, fluorescence | 16–18 |
| HER2 and HER3 | SPECT | 19 |
| TF and CD105 | PET/fluorescence | 20 |
| EGFR and CD105 | PET/fluorescence | 21 |
| EGFR and integrin αvβ3/β5 | MRI | 22 |
| CD3ε and CEA | Fluorescence | 23 |
| CD3ε and EpCAM | PET/fluorescence | 24 |
| CD3ε and NY-ESO-1 | Fluorescence | 25 |
| Nanoparticle-based | ||
| Targets | ||
| CD44 and integrin αvβ3 | Fluorescence | 27,28 |
| FR and integrin αvβ3 | Fluorescence | 29 |
| FR and CD44 | Fluorescence | 30 |
| CD44 and GAR | Fluorescence | 33 |
| FR and integrin αvβ3 | Fluorescence and US | 34 |
| Neurokinin-1 and integrin αvβ3 | SPECT and MRI | 35 |
| P-selectin and integrin αvβ3 | Fluorescence | 36 |
TF = tissue factor; CEA = carcinoembryonic antigen; US = ultrasound; FR = folate receptor.
PEPTIDE-BASED AGENTS
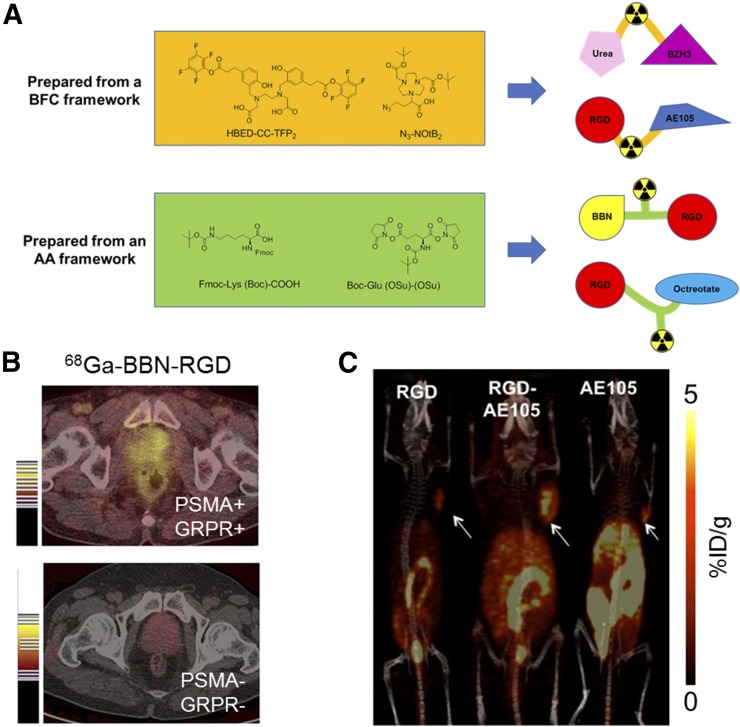
Peptide-based heterodimeric tracers have played an important role in dual-targeted molecular imaging. Although reports have been made with other modalities (6), herein we focus on PET imaging with these heterodimers, as they may be most readily translated to the clinic. The major synthetic strategies for these peptide-based agents can be categorized as using either bifunctional chelators (BFCs) or amino acids as frameworks. The major advantage of using a BFC is that the BFC itself serves as both the linker and the chelator, simplifying the synthetic procedure (Fig. 1A). Eder’s group reported N,N′-bis(2-hydroxy-5-(carboxyethyl)benzyl)ethylenediamine-N,N′-diacetic acid ([HBED-CC]TFP2) as an ideal BFC for constructing peptide-based heterodimeric probes, as it bears two leaving groups for nucleophilic attack by primary amines at both sides of the chelator (7). Another NOTA-based BFC, 4-azido-2-(4,7-bis(2-(tert-butoxy)-2-oxoethyl)-1,4,7-triazonan-1-yl)butanoic acid ([N3-NOtB2]), was developed by Zeng’s group (8). Unlike (HBED-CC)TFP2, which possesses two identical reactive groups, N3-NOtB2 contains two different reactive groups including an N3 group for click chemistry and a COOH group for amide coupling, offering selectivity for conjugation with two different peptides. In addition to BFCs, amino acids such as lysine (9) and glutamate (10) have been developed as frameworks, providing flexibility to select an appropriate chelator based on the radioisotope to fulfill different experimental requirements.
FIGURE 1.
(A) Bifunctional chelator and amino acid frameworks for preparing peptide-based dual-targeted imaging agents. (B) PET imaging of 68Ga-BBN-RGD in a GRPR- and integrin αvβ3–positive clinical prostate cancer patient (top) and doubly negative patient (bottom) (13). (C) PET images of U87MG-bearing mice (arrows) at 4 h after injection of indicated 64Cu-labeled tracers (8).
One example of a peptide-based PET tracer is the RGD-BBN (RGD = Arg-Gly-Asp; BBN = bombesin) heterodimer that was initially developed by Li et al. Using the glutamate-based framework, the resulting dimeric RGD-BBN could simultaneously bind both gastrin-releasing peptide receptor (GRPR) and integrin αvβ3 (11). Both 18F- (12) and 64Cu- (11) labeled RGD-BBN heterodimers were successfully prepared and evaluated in PC-3 xenograft mouse models. Very recently, the dimeric RGD-BBN probe was 68Ga-labeled, and the resulting PET tracer has been successfully used for clinical PET imaging of prostate cancer patients (13), showing clear uptake in tumors with an SUVmax near 4.5 for primary tumors and 6.3 for metastatic lymph nodes (Fig. 1B).
Another recent example is the RGD-AE105 heterodimer that was prepared by Gai et al., using the BFC framework N3-NOtB2 (8). The prepared RGD-AE105 tracer targets both the integrin αvβ3 and the urokinase-type plasminogen activator receptor simultaneously. Saturation binding assay results revealed a smaller equilibrium dissociation constant and larger receptor density for the RGD-AE105 heterodimer than for the two corresponding monomers, indicating the increased binding avidity achieved by the RGD-AE105 heterodimer. The in vivo imaging results, obtained from mice bearing U87MG xenografts (overexpressing both targets), showed the superior tumor uptake of the heterodimer (3.27 percentage injected dose per gram [%ID/g]) compared with that of corresponding RGD or AE105 monomers (1.55 %ID/g for RGD and 1.73 %ID/g for AE105), demonstrating the potential for integrin αvβ3 and urokinase plasminogen activator receptor dual-targeted PET imaging (Fig. 1C). The other two reported RGD-containing bispecific tracers are RGD-octreotate, targeting the somatostatin receptor subtype 2 (9), and RGD-GE11, targeting EGFR (14). The frequent use of RGD for preparing heterodimers suggests its excellent tumor-targeting properties and good pharmacokinetic profile.
Other bispecific agents not containing the RGD motif have also been reported. One representative example is a heterodimer developed by Kopka’s group, targeting both prostate-specific membrane antigen (PSMA) and GRPR (7). Specifically, a urea-based PSMA inhibitor was conjugated with the nonapeptide BZH3 targeting GRPR, using (HBED-CC)TFP2 to generate this heterodimer. Further in vivo evaluation revealed its promising uptake in both PSMA-positive and GRPR-positive tumors (5.4 %ID/g for PSMA-positive and 3.3 %ID/g for GRPR-positive tumors), which suggests application in prostate cancer detection, since both PSMA and GRPR were found to be expressed at a high level in prostate cancer cells.
ANTIBODY- AND ANTIBODY FRAGMENT–BASED AGENTS
With their excellent target specificity, antibody- and antibody fragment–based bispecific molecular imaging agents have gained popularity in the last decade. The synthesis mechanisms for these tracers have been thoroughly outlined elsewhere (15). In general, these agents may fall into two classes: those that target two antigens on cancer cells, and those that target a cancer cell and another type as well. The advantages of dually targeting cancer cells are clear, enabling enhanced specificity and higher image quality. This strategy has been preclinically applied to radionuclide-based imaging, fluorescence imaging, and MRI.
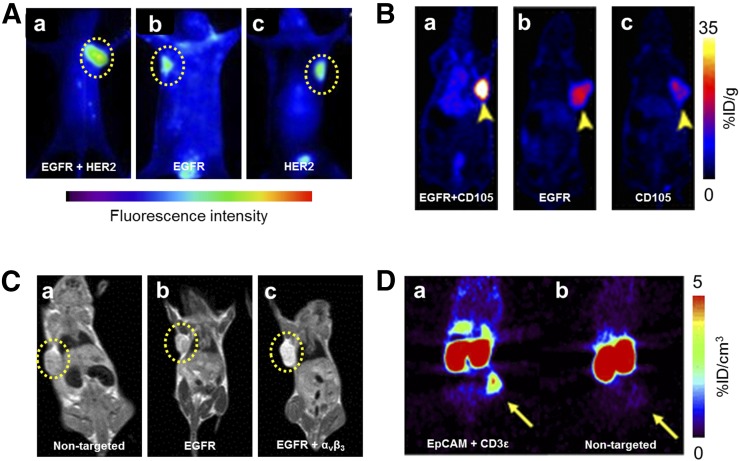
HER2 has been identified as an important therapeutic target in breast cancer and, as such, is also a target of several bispecific imaging agents. Reilly’s group combined a Fab fragment of trastuzumab (targeting HER2) with epidermal growth factor (EGF, targeting its receptor EGFR) through a PEG linker and labeled with 64Cu for PET imaging (16) and an 111In-labeled version for SPECT (17). In EGFR/HER2-positive tumors with the 64Cu-labeled version, the bispecific tracer showed uptake of 4.9 %ID/g, compared with 1.9 %ID/g for only HER2 targeting, or 0.7 %ID/g for the EGF monomer. The inclusion of a long PEG linker in the heterodimer was found to prolong blood circulation time and accordingly increase tumor uptake. Similarly, a multivalent antibody was developed by Ding et al. to target HER2 and EGFR through the conjugation of an anti-EGFR Nanobody to two anti-HER2 Affibody molecules, and imaged through an attached fluorescent dye (Fig. 2A) (18). A SPECT agent was also developed to target both HER2 and HER3 for enhanced breast cancer imaging (19).
FIGURE 2.
(A) Fluorescence imaging of A549 tumors at 8 h after injection of a tracer for EGFR and HER2 (a), EGFR (b), and HER2 (c) (18). (B) PET imaging of U87MG tumors at 36 h after injection shows differences in uptake of agent targeting EGFR and CD105 (a), EGFR (b), and CD105 (c) (21). (C) T1 MR images of BGC-823 tumors 1 h after injection of tracers specific for no targets (a), EGFR alone (b), or both EGFR and αvβ3 (c) (22). (D) PET images 24 h after injection of bispecific EpCAM/CD3ε antibody (a) or isotype control (b) in HT-29 tumor–bearing mice (24).
Other bispecific antibody agents have been fabricated that target not only cancer cells, but also their associated angiogenic vessels. CD105, expressed on neovasculature, has been one of the targets of bispecific agents for PET and fluorescence dual-modality imaging by our group (20,21). Pancreatic cancer models were successfully imaged using a CD105- and tissue factor–targeted bispecific antibody fragment (20), whereas brain cancer was imaged through targeting of CD105 and EGFR with a tracer generated through click chemistry techniques (Fig. 2B) (21). The combination of CD105 and EGFR targeting allowed peak tracer uptake of over 47 %ID/g at 15 h after injection in subcutaneous U87MG tumors, whereas single targeting to either EGFR or CD105 provided significantly lower uptake, demonstrating the synergy that can be achieved through dual targeting. Integrin αvβ3 has also been used for targeting of bispecific imaging agents in combination with EGFR in MRI studies (Fig. 2C) (22).
Antibodies targeting both a cancer cell and an immune cell have shown immense promise in therapeutic settings. The therapeutic mechanisms and effects of bispecific T-cell constructs have been elucidated through direct imaging with the agents preclinically. This has been explored using antibodies targeting CD3ε on T cells along with carcinoembryonic antigen in fluorescence imaging (23), EpCAM for both PET and fluorescence imaging (Fig. 2D) (24), and a cancer testis antigen, NY-ESO-1, for fluorescence imaging (25).
DUAL-TARGETED NANOPARTICLES
Nanoparticles have demonstrated their potential as molecular imaging contrast agents in cancer in nearly every medical imaging modality. Just as with other dual-targeted platforms, the use of two targeting moieties enables nanoparticles to accumulate at a higher level in regions expressing both targets. Nanoparticles are uniquely well-suited for advanced surface modifications, such as conjugation of targeting ligands. This makes dual-targeting with these platforms straightforward in some situations (26). Depending on the nanoparticle and its surface properties, biomolecules may be attached using several strategies, via covalent or noncovalent techniques.
Most reported probes provide contrast in fluorescence imaging, with a few reports in other realms. Similarly, the choices of targeting ligands for dual-targeted nanoparticles have been rather limited. Peptides have been the ligand of choice due to their specificity and small size. Specifically, the targets integrin αvβ3, CD44, and the folate receptor (FR) have been most thoroughly explored.
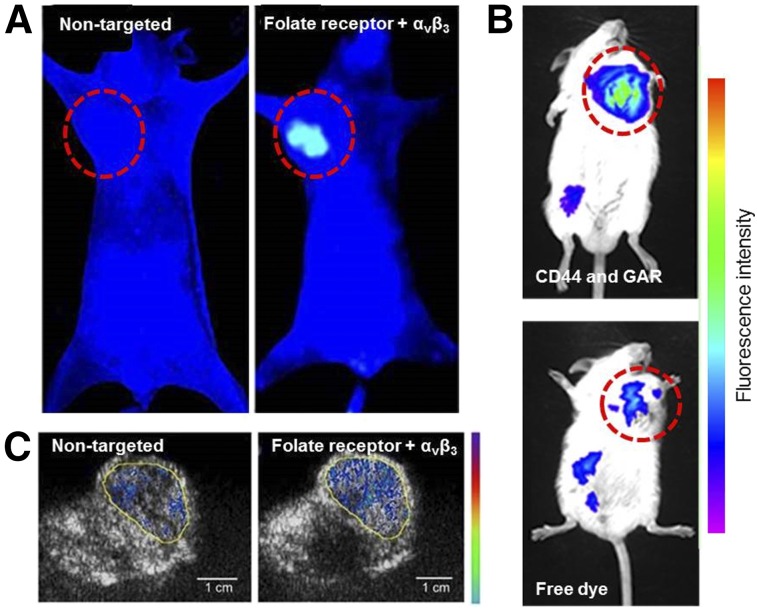
For example, CD44 and integrin αvβ3 have been the targets of two nanoparticle platforms: a core-shell system for gene delivery in melanoma models (27) and solid lipid nanoparticles for delivery of docetaxel (28). Self-assembled hydrogel nanoparticles were dually targeted to integrin αvβ3 and FR, loaded with paclitaxel, and validated in preclinical hepatocellular carcinoma models by Shu et al. (Fig. 3A) (29). Ceramide nanoparticles were developed by Lee et al. and targeted to both CD44 and FR in ovarian cancer (30), and persistent luminescent nanoparticles were targeted to FR and CD44 by Zhao et al. (31). In all these studies, enhanced accumulation in cancerous tissues was demonstrated for dual-targeted nanoparticles over their single or nontargeted counterparts, as evidenced through fluorescence imaging. Other nanoparticles targeting combinations of integrin αvβ3 and EGFR (32) and CD44 along with glycyrrhetinic acid receptors (Fig. 3B) (33) have been imaged using fluorescence as well.
FIGURE 3.
Dual-targeted nanoparticle imaging agents. (A) Fluorescence imaging of tumor-bearing mice 48 h after injection of nontargeted hydrogels (left) or FR and αvβ3-targeted nanoparticles (right) (29). (B) Fluorescence imaging of H22 tumor–bearing mice 12 h after injection of CD44 and GA receptor–targeted nanoparticles (top) and free dye (bottom) (33). (C) Ultrasound-active nanobubbles targeted to FR and αvβ3 (right) accumulated to higher level in tumors than their nontargeted counterparts (left) (34).
Other imaging modalities have also been used to evaluate dual peptide–conjugated nanoparticles. Nanoparticles that were both ultrasound and fluorescence-active were targeted to FR and integrin αvβ3 (Fig. 3C) (34), whereas peptides targeting integrin αvβ3 and neurokinin-1 receptors allowed loaded liposomes to be visualized by SPECT/CT and MRI (35). Similarly, liposomes targeting P-selectin and integrin αvβ3 enabled imaging of metastatic breast cancer models through fluorescence imaging and γ-scintigraphy (36).
CONCLUSIONS AND FUTURE DIRECTIONS
Dual-targeted imaging tracers hold immense potential to increase image quality in various diseases, especially in cancer. Although patients may be grouped into a single category based on disease site, it is becoming increasingly obvious that the heterogeneity of cancer will need to be addressed for successful therapy and personalized/precision medicine. Within a single anatomic site, a particular patient, or even any given lesion, the biomarker expression of a cancer can vary widely. Thus, dual-targeted imaging agents may enable enhanced visualization by providing a larger number of potential binding sites. Additionally, they may find wider application across more patients due to their multiple specificities.
Although the use of two targeting ligands enables clearer visualization of cancerous lesions, this dual-targeting strategy also removes some of the in-depth information that is available from the use of a singly targeted agent. The use of radio- or fluorescently labeled compounds as companion diagnostics is gaining traction for patient stratification and treatment decisions (37); however, using an agent that binds to two different targets will make identification of patients with a given marker difficult, since it is challenging to tease apart which antigen is being recognized by the probe. Therefore, these dual-targeted imaging agents are likely to find success in localizing cancerous lesions, but not perhaps in selecting appropriate treatments for those patients, unless the treatment also uses a bispecific agent. They may, however, be useful for monitoring treatment response, to more readily visualize tumor burden over time.
An imaging tracer with high accumulation in a targeted site could additionally be modified for theranostic applications. This is becoming a common strategy with singly targeted entities—antibodies radiolabeled with imaging nuclides can be exchanged for therapeutic ones (38), additional therapeutic moieties can be attached (18), or the inherent theranostic capabilities of nanoparticles can be optimized (13,27,29,30,33,34). Although a few preclinical therapeutic studies using dual-targeted platforms have been reported to date, this area is certainly waiting for exploration. Targeting of albumin in the bloodstream in addition to a cancer antigen has also been demonstrated to enhance the circulation of agents, enabling increased tumor exposure and uptake of therapeutic agents as well (39).
The bright future of dual-targeting strategies lies in their immense potential for improving imaging quality. A large number of dual-targeted strategies have already been reported in the recent literature, with more added each year. Enabled by this better tumor-to-background contrast, physicians will be able to more clearly visualize a patient’s disease state and make more informed treatment decisions, making great strides toward personalized treatments. This high tumor-to-background should be exploited also for enhanced targeted therapies, whether that is through drug delivery, targeted radiotherapy, or other strategies. We expect that the successful translation of these agents into the clinic will enable earlier, more in-depth, and higher quality noninvasive imaging for cancer patients, thereby greatly improving the care they receive.
DISCLOSURE
This work was supported, in part, by the University of Wisconsin–Madison, the University of Pittsburgh, the National Institutes of Health (NIBIB/NCI P30CA014520, T32GM008505, T32CA009206, R21EB020737, R21EB017317), National Natural Science Foundation of China (No. 81630049), and the American Cancer Society (125246-RSG-13-099-01-CCE, ACS-RSG-17-004-01-CCE). No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Fan G, Wang Z, Hao M, Li J. Bispecific antibodies and their applications. J Hematol Oncol. 2015;8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullard A. FDA approves first bispecific. Nat Rev Drug Discov. 2015;14:7. [DOI] [PubMed] [Google Scholar]
- 3.Cole P. Duligotuzumab. Human anti-EGFR/anti-HER3 MAb, colorectal cancer therapy, head and neck cancer therapy. Drugs Future. 2015;40:167. [Google Scholar]
- 4.Yang Q, Parker CL, McCallen JD, Lai SK. Addressing challenges of heterogeneous tumor treatment through bispecific protein-mediated pretargeted drug delivery. J Control Release. 2015;220:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Y, Chen X. Peptide heterodimers for molecular imaging. Amino Acids. 2011;41:1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eder M, Schafer M, Bauder-Wust U, Haberkorn U, Eisenhut M, Kopka K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate. 2014;74:659–668. [DOI] [PubMed] [Google Scholar]
- 8.Gai Y, Xiang G, Ma X, et al. Universal molecular scaffold for facile construction of multivalent and multimodal imaging probes. Bioconjug Chem. 2016;27:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tateishi U, Oka T, Inoue T. Radiolabeled RGD peptides as integrin αvβ3-targeted PET tracers. Curr Med Chem. 2012;19:3301–3309. [DOI] [PubMed] [Google Scholar]
- 10.Yu Z, Ananias HJ, Carlucci G, et al. An update of radiolabeled bombesin analogs for gastrin-releasing peptide receptor targeting. Curr Pharm Des. 2013;19:3329–3341. [DOI] [PubMed] [Google Scholar]
- 11.Jackson AB, Nanda PK, Rold TL, et al. 64Cu-NO2A-RGD-Glu-6-Ahx-BBN(7-14)NH(2): a heterodimeric targeting vector for positron emission tomography imaging of prostate cancer. Nucl Med Biol. 2012;39:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li ZB, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453–461. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Niu G, Lang L, et al. Clinical translation of a dual integrin αvβ3– and gastrin-releasing peptide receptor–targeting PET radiotracer, 68Ga-BBN-RGD. J Nucl Med. 2017;58:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu HM, Chen JH, Lin KL, Lin WJ. Synthesis of 68Ga-labeled NOTA-RGD-GE11 heterodimeric peptide for dual integrin and epidermal growth factor receptor-targeted tumor imaging. J Labelled Comp Radiopharm. 2015;58:299–303. [DOI] [PubMed] [Google Scholar]
- 15.Luo H, Hong H, Yang SP, Cai W. Design and applications of bispecific heterodimers: molecular imaging and beyond. Mol Pharm. 2014;11:1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon LY, Scollard DA, Reilly RM. 64Cu-labeled trastuzumab fab-PEG24-EGF radioimmunoconjugates bispecific for HER2 and EGFR: pharmacokinetics, biodistribution, and tumor imaging by PET in comparison to monospecific agents. Mol Pharm. 2017;14:492–501. [DOI] [PubMed] [Google Scholar]
- 17.Razumienko E, Dryden L, Scollard D, Reilly RM. MicroSPECT/CT imaging of co-expressed HER2 and EGFR on subcutaneous human tumor xenografts in athymic mice using 111In-labeled bispecific radioimmunoconjugates. Breast Cancer Res Treat. 2013;138:709–718. [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Tian C, Feng S, et al. Small sized EGFR1 and HER2 specific bifunctional antibody for targeted cancer therapy. Theranostics. 2015;5:378–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razumienko EJ, Scollard DA, Reilly RM. Small-animal SPECT/CT of HER2 and HER3 expression in tumor xenografts in athymic mice using trastuzumab Fab-heregulin bispecific radioimmunoconjugates. J Nucl Med. 2012;53:1943–1950. [DOI] [PubMed] [Google Scholar]
- 20.Luo H, England CG, Goel S, et al. ImmunoPET and near-infrared fluorescence imaging of pancreatic cancer with a dual-labeled bispecific antibody fragment. Mol Pharm. 2017;14:1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo H, Hernandez R, Hong H, et al. Noninvasive brain cancer imaging with a bispecific antibody fragment, generated via click chemistry. Proc Natl Acad Sci USA. 2015;112:12806–12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xin X, Sha H, Shen J, Zhang B, Zhu B, Liu B. Coupling GdDTPA with a bispecific, recombinant protein antiEGFRiRGD complex improves tumor targeting in MRI. Oncol Rep. 2016;35:3227–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann S, Perera R, Grimm HP, et al. In vivo fluorescence imaging of the activity of CEA TCB, a novel T-cell bispecific antibody, reveals highly specific tumor targeting and fast induction of T-cell-mediated tumor killing. Clin Cancer Res. 2016;22:4417–4427. [DOI] [PubMed] [Google Scholar]
- 24.Warnders FJ, Waaijer SJ, Pool M, et al. Biodistribution and PET imaging of labeled bispecific T cell-engaging antibody targeting EpCAM. J Nucl Med. 2016;57:812–817. [DOI] [PubMed] [Google Scholar]
- 25.McCormack E, Adams KJ, Hassan NJ, et al. Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother. 2013;62:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conde J, Dias JT, Grazu V, Moros M, Baptista PV, de la Fuente JM. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front Chem. 2014;2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Song L, Yang X, et al. Multifunctional “core-shell” nanoparticles-based gene delivery for treatment of aggressive melanoma. Biomaterials. 2016;111:124–137. [DOI] [PubMed] [Google Scholar]
- 28.Shi S, Zhou M, Li X, et al. Synergistic active targeting of dually integrin alphavbeta3/CD44-targeted nanoparticles to B16F10 tumors located at different sites of mouse bodies. J Control Release. 2016;235:1–13. [DOI] [PubMed] [Google Scholar]
- 29.Shu C, Sabi-Mouka EMB, Wang X, Ding L. Self-assembly hydrogels as multifunctional drug delivery of paclitaxel for synergistic tumour-targeting and biocompatibility in vitro and in vivo. J Pharm Pharmacol. 2017;69:967–977. [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Termsarasab U, Park JH, et al. Dual CD44 and folate receptor-targeted nanoparticles for cancer diagnosis and anticancer drug delivery. J Control Release. 2016;236:38–46. [DOI] [PubMed] [Google Scholar]
- 31.Zhao HX, Yang CX, Yan XP. Fabrication and bioconjugation of BIII and CrIII co-doped ZnGa2O4 persistent luminescent nanoparticles for dual-targeted cancer bioimaging. Nanoscale. 2016;8:18987–18994. [DOI] [PubMed] [Google Scholar]
- 32.Zhang MZ, Li C, Fang BY, et al. High transfection efficiency of quantum dot-antisense oligonucleotide nanoparticles in cancer cells through dual-receptor synergistic targeting. Nanotechnology. 2014;25:255102. [DOI] [PubMed] [Google Scholar]
- 33.Mezghrani O, Tang Y, Ke X, et al. Hepatocellular carcinoma dually-targeted nanoparticles for reduction triggered intracellular delivery of doxorubicin. Int J Pharm. 2015;478:553–568. [DOI] [PubMed] [Google Scholar]
- 34.Luo W, Wen G, Yang L, et al. Dual-targeted and pH-sensitive doxorubicin prodrug-microbubble complex with ultrasound for tumor treatment. Theranostics. 2017;7:452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rangger C, Helbok A, Sosabowski J, et al. Tumor targeting and imaging with dual-peptide conjugated multifunctional liposomal nanoparticles. Int J Nanomedicine. 2013;8:4659–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doolittle E, Peiris PM, Doron G, et al. Spatiotemporal targeting of a dual-ligand nanoparticle to cancer metastasis. ACS Nano. 2015;9:8012–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puranik AD, Kulkarni HR, Baum RP. Companion diagnostics and molecular imaging. Cancer J. 2015;21:213–217. [DOI] [PubMed] [Google Scholar]
- 38.Razumienko EJ, Chen JC, Cai Z, Chan C, Reilly RM. Dual-receptor–targeted radioimmunotherapy of human breast cancer xenografts in athymic mice coexpressing HER2 and EGFR using 177Lu- or 111In-labeled bispecific radioimmunoconjugates. J Nucl Med. 2016;57:444–452. [DOI] [PubMed] [Google Scholar]
- 39.Kelly JM, Amor-Coarasa A, Nikolopoulou A, et al. Dual-target binding ligands with modulated pharmacokinetics for endoradiotherapy of prostate cancer. J Nucl Med. 2017;58:1442–1449. [DOI] [PubMed] [Google Scholar]