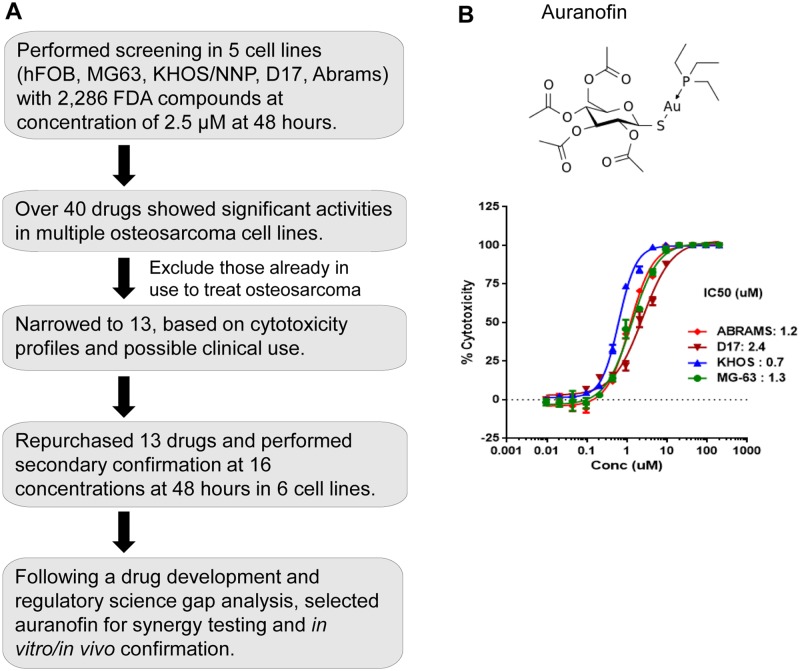
Fig 1. Primary screening of FDA-approved library identifies auranofin as a potential drug for OS therapy.
A. A diagram of the primary screening to select auranofin. B. Auranofin chemical structure (top) and concentration-response curves of auranofin cytotoxic effects on human (MG-63 and KHOS/NP) and canine (Abrams and D17) OS cells (bottom). Graph also includes IC50 values for each cell line. Error bars: means ± S.D. from 3-independent experiments.