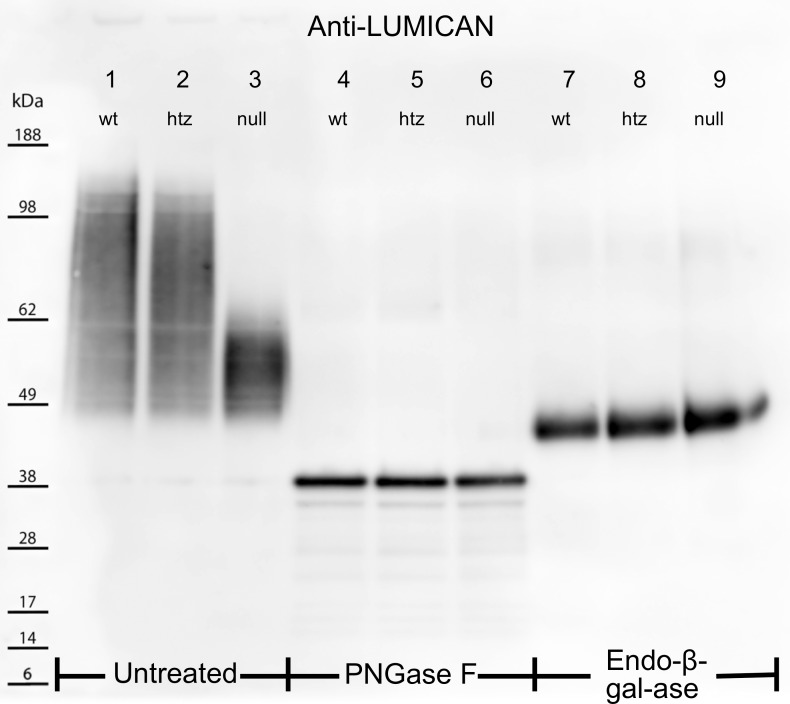
Figure 3.
Electrophoretically separated untreated (lanes 1–3), PNGase F– (lanes 4–6), and Endo-β-Gal-ase–digested (lanes 7–9) corneal extracts were probed for the KS core protein, lumican. Untreated WT and htz samples produced smear patterns from ∼160 to 45 kDa (lanes 1, 2), whereas untreated null extracts showed a more condensed band from 45 to 65 kDa (lane 3). PNGase F cleaved KS-GAG chains from lumican core proteins in lanes 4–6. The migration of lumican into narrow, low-molecular-weight bands at 40 kDa for all three samples indicated the differential smear patterns in the untreated samples were a result of altered KS structures. Lanes 7–9 show lumican localization following cleavage of KS-GAGs at unsulfated Gal residues using Endo-β-Gal-ase. All three samples showed thick bands at ∼46 kDa following this treatment, suggesting sulfation of Gal residues is unaffected by the B3gnt7 mutation.