Fig. 2.
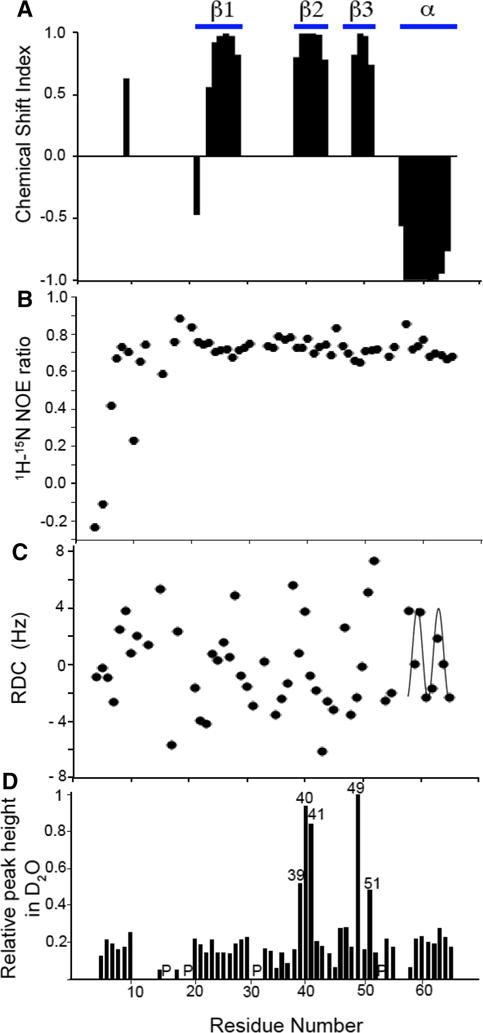
Plots of experimental data measured on monomeric IL-8(1–66) in solution as a function of residue number. a Chemical Shift Index. b 1H/15N heteronuclear NOE. c Residual Dipolar Couplings used to cross-validate the solution NMR structure of monomeric IL-8 (1–66). The helical region of the protein displays a characteristic Dipolar Wave pattern. d Resonance intensities after incubation of IL-8(1–66) in D2O for 1 h. Peak height was measured for all peaks in a 1H/15N HSQC spectrum and normalized to the peak height of residue Leu49. The locations of proline residues are indicated with a ‘P’
