Abstract
Introduction
Fetal hydronephrosis (HN) occurs in approximately 5% of pregnancies and its prognosis depends mainly on the grade of the dilation. We attempted to determine the fate of isolated, unilateral, high-grade HN in children with antenatal diagnosis, emphasizing the risk factors for progression.
Methods
We retrospectively evaluated 424 children (690 kidney units) with antenatal HN in the period between 2010 and 2014. We included only those patients with isolated high-grade HN (Society for Fetal Urology [SFU] Grade 3 or 4). Patients with bilateral HN or unilateral HN associated with dilated ureter or reflux and patients with missed followup were excluded. The prognosis of HN (whether improved, stabilized, or progressed) and the need for surgical intervention in this subset of patients was evaluated.
Results
A total of 44 children (34 boys and 10 girls) were identified. Ultrasounds showed SFU Grade 3 HN in 24 (54%) and SFU Grade 4 HN in 20 (46%). After a mean followup of three years (range 1–5), 10 children (23%) needed surgical intervention; four Grade 3 HN (16%) and six Grade 4 HN (30%). The majority of children with differential renal function (DRF) ≥40% (69.5%) were stable or improved. Five girls (50%) and five boys (17%) progressed and required surgical intervention. No patient with a renal pelvis anteroposterior diameter (APD) <1.5 cm needed surgical intervention.
Conclusions
Infants with isolated, unilateral, high-grade HN might be managed conservatively. Male gender, DRF ≥40%, SFU Grade 3 HN, and APD <1.5 cm were favourable prognostic factors.
Introduction
Antenatal hydronephrosis (HN) occurs in 1–5% of all pregnancies. 1 Once diagnosed, the debate will arise whether it is obstructive or non-obstructive in nature, harmful to the kidney or not, and whether any prenatal or postnatal surgical intervention will be required.2
Multiple etiologies of antenatal HN have been reported, including transient HN, pelviureteric junction obstruction, vesicoureteral reflux (VUR), posterior urethral valves, or other anomalies.3 Exclusion of the aforementioned disorders define the term isolated antenatal HN. 4
The exact definition of significant antenatal HN is evolving. Multiple grading systems and parameters were used to define this entity; however, the Society for Fetal Urology (SFU) grading system and the measurement of antero-posterior diameter (APD) of the renal pelvis were among the most commonly used.1–3
High-grade antenatal HN will usually require extensive evaluation and strict followup. This might require the use of continuous antibiotic prophylaxis (CAP), further evaluation, longer followup period, and possibly surgical intervention, depending on the underlying etiology.4 On the other hand, low-grade HN is usually benign in nature and about 50% of fetuses and infants diagnosed with this entity might show complete resolution on followup.5
In our study, we examined the natural history of isolated, high-grade antenatal HN. Our aim was to assign criteria for possible differentiations between cases that deserve observation and those that might necessitate surgical intervention in the hope of aiding the physician in parental counselling and risk stratification.
Methods
After obtaining the approval of our institutional review board, a retrospective study design was constructed. We identified patients from our registry of prenatal congenital anomalies in the period from January 2010 to December 2014.
The first postnatal renal ultrasound (US) was obtained during the first week of life in all patients, beyond the first 72 hours. The SFU grading system was used to classify all patients, and US images were again reviewed by two physicians, a pediatric radiologist and a pediatric urologist, to minimize inter-reviewer variability. If physicians disagreed, the higher grade was taken. Patients with no available images were excluded from the study. Additionally, measurement of the APD taken at the renal hilum in supine position was recorded.
All patients included in the study were investigated by voiding cystourethrography and cases with vesicoureteral reflux (VUR), posterior urethral valves, or ureterocele were excluded.
The first diuretic renography (DR), using mercaptoacetyltriglycine (MAG-3) was performed on all patients 8–12 weeks after birth. A standardized DR was performed universally in all patients. MAG-3 was injected using a dose of 0.1 mCi/kg (minimum 1 mCi, maximum 5 mCi). Differential renal function (DRF) was calculated from the second-minute image after drawing tight regions of interest and using a standardized technique for background subtraction. When the entire dilated collecting system was filled with the radiotracer, 1 mg/kg of furosemide was administered (minimum 1 mg, maximum 40 mg); dynamic images were obtained for 30 minutes and T1/2s were calculated post-diuresis. In cases of a flat or rising curve, a T1/2 of 100 minutes was assigned. Drainage was classified as good if T1/2 was <20 minutes; fair if T1/2 was >20 minutes and the drainage curve was descending; or poor if T1/2 could not be counted and there was a rising drainage curve.
We identified 424 patients diagnosed with prenatally detected HN. The first performed postnatal ultrasound was used as a base for selection of patients with HN and only those patients with isolated, unilateral, high-grade HN (SFU Grade 3 and 4) were included. We excluded any patient with other anomalies, namely duplication anomaly, multicystic dysplastic kidney, dilated ureter, VUR, ureterocele, ectopic ureter, urinary bladder diverticulum, thickened bladder wall, or posterior urethral valves.
Of 424 patients (690 kidney units), 275 patients (550 kidney units) were excluded due to bilaterality (any grade of HN in the contralateral kidney). Of the 149 patients with unilateral HN, 105 cases were excluded — 66 with low-grade HN and 39 found to be associated with other urological anomalies.
The number of urinary tract infections (UTIs) in the included cohort was determined by reviewing urine culture microbiological data. A UTI was defined as bacterial growth of 105 CFU/ml when a clean catch technique was used and 104 CFU/ml if catheterized urine sample was used. All patients included in the study were not initially on CAP, and all males included were circumcised at early neonatal period due to religious concerns.
During followup, all patients underwent regular renal ultrasonographic examination in intervals that ranged from 1–6 months (mean = 3 months), during the first year of life from of 3–9 months thereafter. All patients included in the study had one baseline DR and further studies were performed 6–12 months apart during followup.
By the end of followup period, all included patients were categorized as improved, stabilized, or deteriorated based on radiological findings in renal US and DR. Improved cases showed downgrading of HN grade on renal US and/or improved drainage on DR. Stabilized cases had stable HN grade on US and stable DRF ≥40% on DR. Indications for intervention were worsening of HN, deteriorated DRF by more than 10%, DRF below 40% with an obstructive curve in DR, or recurrent febrile UTI. All cases were operated upon within the first year of life.
Statistical analysis was performed using SPSS version 21 (SPSS Inc., Chicago, IL, U.S.). We used binary and logistic regression analyses. Values are shown as mean + standard deviation (SD) unless otherwise reported. Kaplan-Meier estimates were used to show the effect of HN grade, side, and gender on the fate of HN. All tests were two-sided. A p<0.05 was considered statistically significant.
Results
Our study included 44 patients with isolated, unilateral, high-grade HN (SFU Grade 3 and 4) who met our study criteria for final analysis. Patient demographics are shown in Table 1. The followup period ranged from 2–7 years (mean = 4 years). At the end of followup, 10 infants (23%) had worsening of HN that required surgical intervention, four with Grade 3 (16%) and six with Grade 4 (30%). Improvement of HN was evident in 12 patients (27%), while HN continued to be stable in the remaining 22 (50%).
Table 1.
Patient demographics
| Unilateral isolated high-grade HN | n=44 | % | |
|---|---|---|---|
| Gender | Male | 34 | 77 |
| Female | 10 | 23 | |
| Laterality | Right | 14 | 32 |
| Left | 30 | 68 | |
| SFU | Grade 3 | 24 | 54.5 |
| Grade 4 | 20 | 45.5 | |
| APD | >15 mm | 19 | 43 |
| ≤15 mm | 25 | 57 | |
| DRF | ≥40% | 35 | 79.5 |
| <40% | 9 | 20.5 | |
APD: anteroposterior diameter; DRF: differential renal function; HN: hydronephrosis; SFU: Society for Fetal Urology.
Using Kaplan-Meier estimates, the effect of gender on progression of HN was statistically significant, as it showed that 50% of girls needed intervention compared to only 17% of boys (p=0.034) (Fig. 1).
Fig. 1.
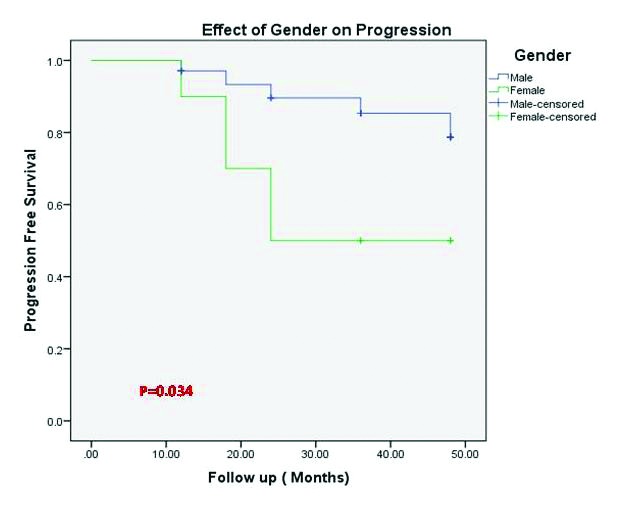
Kaplan-Meier curve showing incidence of progression of hydronephrosis stratified by gender.
All patients who required surgical intervention had APD of >15 mm while, none with APD ≤15 mm needed intervention, and that was statistically significant (p=0.001) (Fig. 2). The mean APD for deteriorated cases was 25 mm.
Fig. 2.
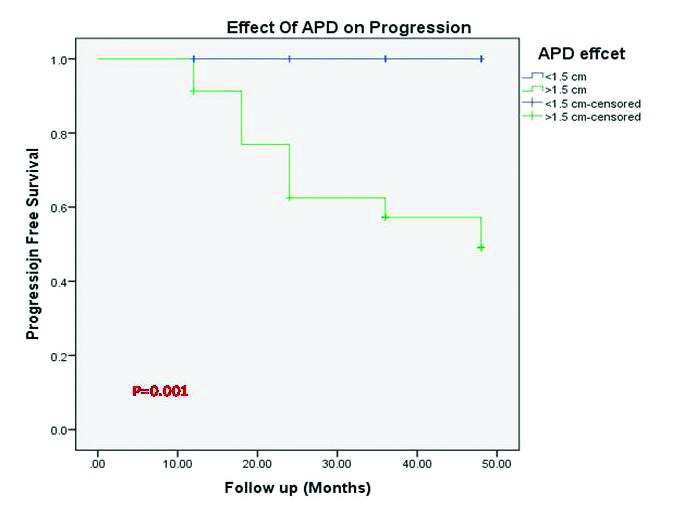
Kaplan-Meier curve showing incidence of progression of hydronephrosis stratified by anteroposterior diameter (APD).
Of patients with DRF ≥40%, only 20% required intervention, whereas in those with DRF <40%, 33.3% needed intervention; the difference was also statistically significant (p =0.004) (Fig. 3).
Fig. 3.
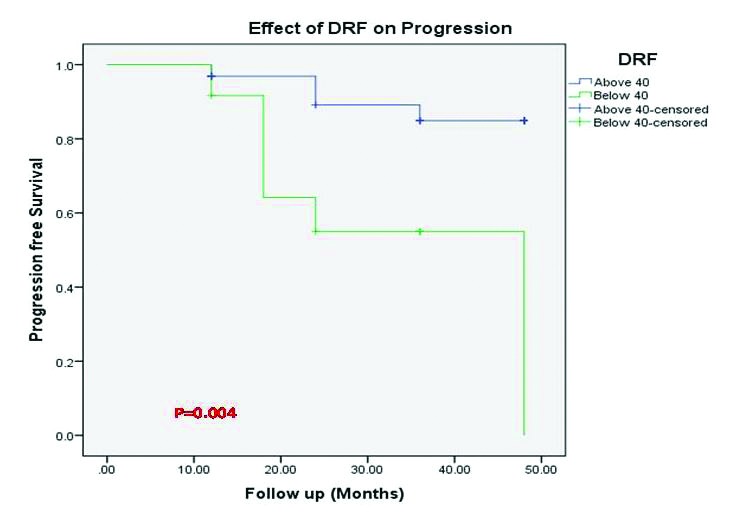
Kaplan-Meier curve showing incidence of progression of hydronephrosis stratified by differential renal function (DRF).
UTIs occurred in four (9%) children in our cohort; none of them were initially on CAP and all were Grade 4 HN and needed surgery. The isolated microorganisms were pseudomonas aeruginosa in two patients, E. coli in one, and klebsiella pneumoniae in another. Furthermore, all patients who underwent pyeloplasty showed improved HN and non-obstructive curve on DR postoperatively, with a mean followup period of two years; none of them developed UTI.
Discussion
Improvements in healthcare facilities and US technology made fetal HN and other genitourinary anomalies more easily diagnosed during maternal antenatal screening. In the past, these anomalies were diagnosed later in life, when they are symptomatic or complicated.6
HN is an anatomical term and is not equivalent to obstruction, nor an indicator of renal function impairment. It may show improvement or spontaneous resolution and no single test can predict future obstruction.7 Because of these uncertainties and an incomplete understanding of the natural history of this anomaly, many controversies exist in how to manage antenatal HN.8 Also, the impact of followup protocols and interventions in this type of urinary tract anomaly in terms of patient outcome needs to be further clarified. The controversies exist more with high-grade, antenatal HN, as it carries a significant risk of uropathology and renal unit function deterioration.9 We aimed to determine which of our cohort of patients with unilateral, high-grade HN required intervention in comparison to those who continued to be followed up conservatively.
In our study, after a mean followup period of 48 months, 77% of patients could be managed conservatively and only 23% needed surgical intervention. The incidence of surgical intervention was two-fold higher in Grade 4 HN, which corresponds with previous study results.10–12
Coelho et al reported that 56% of infants with SFU Grade 4 HN required surgical intervention, whereas only 5% of those with SFU Grade 3 HN did.10 A meta-analysis done by Sidhu et al showed that isolated antenatal HN was five times more likely to stabilize if associated with SFU Grade 1–2 or APD <12 mm than with SFU Grade 3 and 4.11 Furthermore, Homsy et al reported that 14% of antenatal HN SFU Grade 3 and 32% of Grade 4 showed further deterioration and, hence, surgery was indicated.12 Meanwhile, Ross et al found that the majority of infants with high-grade HN do not require early surgical intervention. Only 44% of kidneys with Grade 4 HN and 4% of those with Grade 3 HN underwent early surgery for high-grade obstruction.13 In contrast, Chertin et al have shown that 50% of children followed conservatively went on to surgery.14 However, one must recognize that the criteria for surgical intervention are variable and may be affected by the surgeons’ and parents’ wishes.
In our study, we investigated the effect of APD on the fate of isolated, unilateral, high-grade, antenatal HN. We found that no patient with APD <15 mm needed intervention while 52% of patients with APD >15 mm required surgery.
Although APD measurement accuracy is debatable itself, this commonly used ultrasonographic parameter in the followup examination of hydronephrotic kidneys is prone to hydration effects over 10% change in the maximum AP diameter of the pelvis. However, the pelvic AP diameter at renal hilum is less affected and seems to be independent of hydration status, thus it is more reliable than maximum AP diameter as a followup tool in HN.15
Masson et al reported that all patients with APD >15 mm went for surgery, but all had obstructed curve on diuretic renography.16 In their study of 178 neonates, Sharifian et al reported 42 patients (23%) required surgery.17 The area under the curve for APD to predict the need for surgery was 0.925, with an APD cutoff of 15 mm. The diagnostic value of APD for determining the need for surgery was determined by sensitivity and specificity of 95.2% and 73.5%, respectively.17
St. Aubin et al investigated the prenatal APD effect and concluded that antenatal HN SFU Grades 3 or 4 and APD ≥9 mm on third trimester US were associated with an increased risk of surgical intervention in the postnatal period. Patients with an SFU Grade 4 progressed to intervention at the fastest rate.18
Kim et al concluded that an APD cutoff of 5, 8, and 10 mm during the second, early third, and late third trimesters, respectively, is more specific in predicting the need for postnatal surgical intervention in the Korean population.19
In our study, most infants with DRF ≥40% were improved or stabilized, while in those with DRF <40%, one-third required surgical intervention. Chertin et al concluded that conservative management is appropriate for infants with DRF exceeding 40% even with an obstructive pattern on DR.20 Multiple authors go with conservative management in this group of patients, but recommend a serial US and repeated DR if there is persistent or progressive HN or parenchymal thinning.21,22
We also investigated the effect of gender on the fate of high-grade HN in our cohort. We found that 50% of the females and only 17% of males deteriorated and required intervention, and that was statistically significant. We could not find in the literature any effect of gender on the natural history of antenatal HN. That may be due to the fact that male fetuses have larger renal pelvic APD and smaller bladder volumes than females. This gender difference in morphology indicates different urinary tract development between male and female fetuses, which likely accounts for gender difference in the urinary tract anomalies detected during the neonatal period.23
The incidence of UTI among our patients was 9%. No patient with Grade 3 antenatal HN developed UTI; only those with Grade 4 HN had this augmented risk. Of note, UTI events may be underestimated, as recording events were dependent on family physician reports and symptoms of UTI; no routine urine cultures were conducted in most of our cohort unless symptomatic. Coelho et al reported that in a cohort of prenatal HN, the incidence of UTI during followup was higher among infants with moderate/severe renal pelvic dilatation (20%) than among patients with mild dilatation (7.8%).10
Braga et al reported the results of multiple meta-analyses that showed UTI was higher in cases with high-grade antenatal HN than low-grade.24 It showed the pooled UTI rates in patients with low-grade antenatal HN were similar regardless of CAP status.4,25–27 In the high-grade HN group, infants on CAP had a significantly lower UTI rate vs. those not on CAP. Because of this result, most recommended CAP for patients with high-grade antenatal HN.28,29
American Urological Association update series on prenatal diagnosis of urological disease (2009) stated that although CAP is generally recommended for severe (Grades 3 and 4) HN, the practice is not evidence-based.28 Multiple recent studies showed that there is recovery of kidney function even in cases with delayed surgery in patients with high-grade antenatal HN and with evidence of obstruction.4,20,22
The limitations of our study include its retrospective nature and its fairly small number of patients with highly selective inclusion criteria. No biomarkers for obstruction were involved and the long-term outcome was not addressed in the study. Future prospective, well-designed studies are encouraged to answer our research question about the dilemma of high-grade HN.
Conclusion
Patients with isolated, high-grade antenatal HN can still be managed conservatively and we can avoid unnecessary surgery without endangering the kidney function. For parental counselling, male gender, SFU Grade 3, DRF >40%, and APD<15 mm are all favourable prognostic factors. Close observation is a needed, especially in the first year of life.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Lee RS, Cendron M, Kinnamon DD, et al. Antenatal hydronephrosis as a predictor of postnatal outcome: A meta-analysis. Pediatrics. 2006;118:586–93. doi: 10.1542/peds.2006-0120. https://doi.org/10.1542/peds.2006-0120. [DOI] [PubMed] [Google Scholar]
- 2.Riccabona M. Assessment and management of newborn hydronephrosis. World J Urol. 2004;22:73–8. doi: 10.1007/s00345-004-0405-0. https://doi.org/10.1007/s00345-004-0405-0. [DOI] [PubMed] [Google Scholar]
- 3.Mallik M, Watson AR. Antenatally detected urinary tract abnormalities: More detection but less action. Ped Nephrol. 2008;23:897–904. doi: 10.1007/s00467-008-0746-9. https://doi.org/10.1007/s00467-008-0746-9. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AM, Phan V, Geary DF, et al. Outcome of isolated antenatal hydronephrosis. Arch Pediatr Adolesc Med. 2004;158:38–40. doi: 10.1001/archpedi.158.1.38. https://doi.org/10.1001/archpedi.158.1.38. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa JA, Chow JS, Benson CB, et al. Postnatal longitudinal evaluation of children diagnosed with prenatal hydronephrosis: Insights in natural history and referral pattern. Prenat Diagn. 2012;32:1242–9. doi: 10.1002/pd.3989. https://doi.org/10.1002/pd.3989. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Hou Y, Niu ZB, et al. Long-term followup and management of prenatally detected, isolated hydronephrosis. J Pediatr Surg. 2010;45:1701–6. doi: 10.1016/j.jpedsurg.2010.03.030. https://doi.org/10.1016/j.jpedsurg.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Hafez AT, McLorie G, Bagli D, et al. Analysis of trends on serial ultrasound for high-grade neonatal hydronephrosis. J Urol. 2002;168:1518–21. doi: 10.1016/S0022-5347(05)64508-9. https://doi.org/10.1016/S0022-5347(05)64508-9. [DOI] [PubMed] [Google Scholar]
- 8.Shokeir AA, Nijman RJ. Antenatal hydronephrosis: Changing concepts in diagnosis and subsequent management. BJU Int. 2000;85:987–94. doi: 10.1046/j.1464-410x.2000.00645.x. https://doi.org/10.1046/j.1464-410x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 9.Karakurt N, Beşbaş N, Bozacı AC, et al. Antenatal hydronephrosis: A single centre’s experience and followup strategies. Turk J Pediatr. 2015;57:560–5. [PubMed] [Google Scholar]
- 10.Coelho GM, Bouzada MC, Pereira AK, et al. Outcome of isolated antenatal hydronephrosis: A prospective cohort study. Pediatr Nephrol. 2007;22:1727–34. doi: 10.1007/s00467-007-0539-6. https://doi.org/10.1007/s00467-007-0539-6. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu G, Beyene J, Rosenblum ND. Outcome of isolated antenatal hydronephrosis: A systematic review and meta-analysis. Pediatr Nephrol. 2006;21:218–24. doi: 10.1007/s00467-005-2100-9. https://doi.org/10.1007/s00467-005-2100-9. [DOI] [PubMed] [Google Scholar]
- 12.Homsy YL, Saad F, Laberge I, et al. Transitional hydronephrosis of the newborn and infant. J Urol. 1990;144:579–83. doi: 10.1016/s0022-5347(17)39527-7. https://doi.org/10.1016/S0022-5347(17)39527-7. [DOI] [PubMed] [Google Scholar]
- 13.Ross SS, Kardos S, Krill A, et al. Observation of infants with SFU Grades 3–4 hydronephrosis: Worsening drainage with serial diuresis renography indicates surgical intervention and helps prevent loss of renal function. J Pediatr Urol. 2011;7:266–71. doi: 10.1016/j.jpurol.2011.03.001. https://doi.org/10.1016/j.jpurol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Chertin B, Pollack A, Koulikov D, et al. Does renal function remain stable after puberty in children with prenatal hydronephrosis and improved renal function after pyeloplasty? J Urol. 2009;182:1845–8. doi: 10.1016/j.juro.2009.03.008. https://doi.org/10.1016/j.juro.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Cost NG, Prieto JC, Wilcox DT. Screening ultrasound in followup after pediatric pyeloplasty. Urology. 2010;76:175–9. doi: 10.1016/j.urology.2009.09.092. https://doi.org/10.1016/j.urology.2009.09.092. [DOI] [PubMed] [Google Scholar]
- 16.Masson P, De Luca G, Tapia N, et al. Postnatal investigation and outcome of isolated fetal renal pelvis dilatation. Arch Pediatr. 2009;16:1103–10. doi: 10.1016/j.arcped.2009.05.008. https://doi.org/10.1016/j.arcped.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Sharifian M, Esfandiar N, Mohkam M, et al. Diagnostic accuracy of renal pelvic dilatation in determining outcome of congenital hydronephrosis. Iran J Kidney Dis. 2014;8:26–30. [PubMed] [Google Scholar]
- 18.St. Aubin M, Willihnganz-Lawson K, Varda BK, et al. Society for Fetal Urology recommendations for postnatal evaluation of prenatal hydronephrosis — will fewer voiding cystourethrograms lead to more urinary tract infections? J Urol. 2013;190:1456–61. doi: 10.1016/j.juro.2013.03.038. https://doi.org/10.1016/j.juro.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Jung HJ, Lee HY, et al. Diagnostic value of anteroposterior diameter of fetal renal pelvis during second and third trimesters in predicting postnatal surgery among Korean population: Useful information for antenatal counseling. Urology. 2012;79:1132–7. doi: 10.1016/j.urology.2012.01.007. https://doi.org/10.1016/j.urology.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Chertin B, Pollack A, Koulikov D, et al. Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: Lessons learned after 16 years of followup. Eur Urol. 2006;49:734–8. doi: 10.1016/j.eururo.2006.01.046. https://doi.org/10.1016/j.eururo.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 21.Ransley PG, Dhillon HK, Gordon I, et al. The postnatal management of hydronephrosis diagnosed by prenatal ultrasound. J Urol. 1990;144:584–7. doi: 10.1016/s0022-5347(17)39528-9. https://doi.org/10.1016/S0022-5347(17)39528-9. [DOI] [PubMed] [Google Scholar]
- 22.Areses Trapote R, Urbieta Garagorri MA, Ubetagoyena Arrieta M, et al. Severe primary congenital unilateral hydronephrosis. A review of 98 cases. An Pediatr (Barc) 2006;64:11–20. doi: 10.1016/s1695-4033(06)70003-0. https://doi.org/10.1016/S1695-4033(06)70003-0. [DOI] [PubMed] [Google Scholar]
- 23.Bassanese G, Travan L, D’Ottavio G, et al. Prenatal anteroposterior pelvic diameter cutoffs for postnatal referral for isolated pyelectasis and hydronephrosis: More is not always better. J Urol. 2013;190:1858–63. doi: 10.1016/j.juro.2013.05.038. https://doi.org/10.1016/j.juro.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Braga LH, Mijovic H, Farrokhyar F, et al. Antibiotic prophylaxis for urinary tract infections in antenatal hydronephrosis. Pediatrics. 2013;131:e251–61. doi: 10.1542/peds.2012-1870. https://doi.org/10.1542/peds.2012-1870. [DOI] [PubMed] [Google Scholar]
- 25.Brophy MM, Austin PF, Yan Y, Coplen DE. Vesicoureteral reflux and clinical outcomes in infants with prenatally detected hydronephrosis. J Urol. 2002;168:1716–9. doi: 10.1097/01.ju.0000026907.65728.6e. https://doi.org/10.1016/S0022-5347(05)64396-0. [DOI] [PubMed] [Google Scholar]
- 26.Wollenberg A, Neuhaus TJ, Willi UV, et al. Outcome of fetal renal pelvic dilatation diagnosed during the third trimester. Ultrasound Obstet Gynecol. 2005;25:483–8. doi: 10.1002/uog.1879. https://doi.org/10.1002/uog.1879. [DOI] [PubMed] [Google Scholar]
- 27.De Kort EH, Bambang Oetomo S, Zegers SH. The long-term outcome of antenatal hydronephrosis up to 15 millimetres justifies a noninvasive postnatal followup. Acta Paediatr. 2008;97:708–13. doi: 10.1111/j.1651-2227.2008.00749.x. https://doi.org/10.1111/j.1651-2227.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- 28.Estrada CR, Peters CA, Retik AB, et al. Vesicoureteral reflux and urinary tract infection in children with a history of prenatal hydronephrosis — should voiding cystourethrography be performed in cases of postnatally persistent grade 2 hydronephrosis? J Urol. 2009;181:801–6. doi: 10.1016/j.juro.2008.10.057. https://doi.org/10.1016/j.juro.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 29.Roth CC, Hubanks JM, Bright BC, et al. Occurrence of urinary tract infection in children with significant upper urinary tract obstruction. Urology. 2009;73:74–8. doi: 10.1016/j.urology.2008.05.021. https://doi.org/10.1016/j.urology.2008.05.021. [DOI] [PubMed] [Google Scholar]


