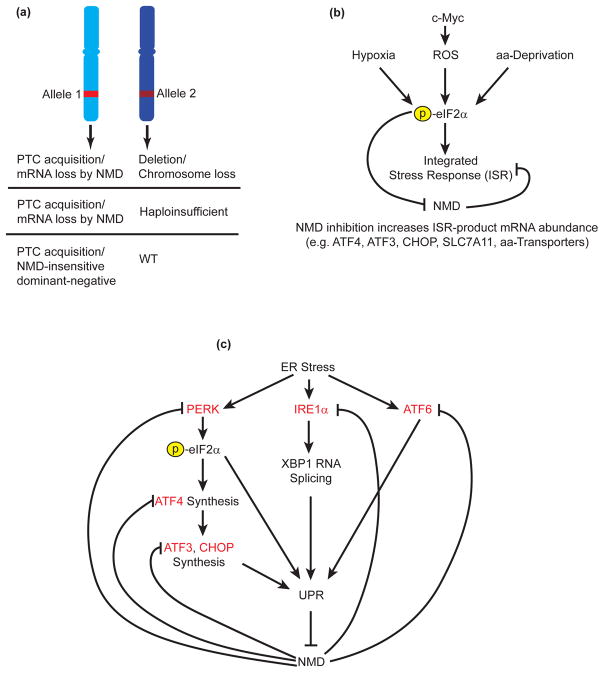
Fig. 1.
The roles that NMD plays in cancer. (a) Tumor-suppressor gene function can be inactivated by NMD. PTC introduction that leads to the loss of tumor-suppressor mRNA via NMD can be combined with either a deletion or chromosomal loss of the wild-type (WT) version, or the remaining wild-type version may be haploinsufficient. Alternatively, PTC acquisition in a region that fails to trigger NMD may lead to production of a dominant-negative allele that interferes with wild-type function. (b) NMD is involved in adaptation to the tumor microenvironment. Stresses in the microenvironment such as hypoxia, production of reactive oxygen species (ROS), and amino acid (aa)-deprivation, lead to phosphorylation of eIF2α and the initiation of the integrated stress response (ISR) in an attempt to adapt to these insults. One outcome of eIF2α phosphorylation is the inhibition of NMD by a poorly understood mechanism. Among the endogenous NMD targets upregulated are components of the ISR. (c) Mutual regulation by the unfolded-protein response (UPR) and NMD. Endoplasmic reticulum (ER) stress causes activation of three ER sensors—PERK, IRE1α, and ATF6. PERK activation leads to phosphorylation of eIF2α and a decrease in global protein synthesis as well as production of ATF4, which upregulates ATF3 and CHOP. These gene products are necessary for the initiation of the UPR, where the cell attempts to increase its protein-folding capacity. IRE1α activation leads to cytoplasmic splicing of XBP1 RNA and activation of the UPR. ATF6 cleavage likewise leads to activation of the UPR. UPR activation attenuates NMD, upregulating a suite of endogenous NMD targets that include UPR components (red). This mutual regulation controls the timing and magnitude of the UPR.