Figure 1. Increased Morbidity and Mortality of Aged Mice in Response to Secondary S. pneumoniae Infection.
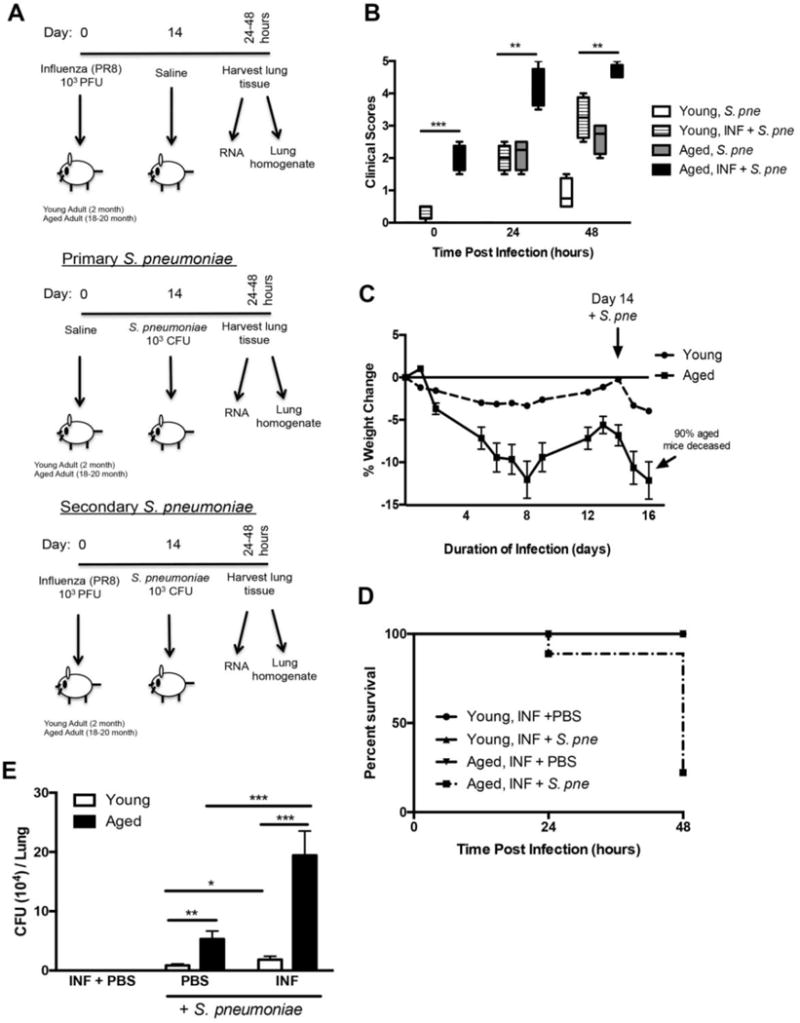
(A) Experimental layout of primary influenza (day 0 instillation with 103 PFU influenza A/PR/8/34 H1N1 (PR8) followed by day 14 instillation with PBS), primary S. pneumoniae (day 0 instillation with PBS followed by day 14 instillation with 103 CFU of ATCC 6303), and secondary S. pneumoniae (day 0 instillation with PR8 103 PFU followed by day 14 instillation with 103 CFU of ATCC 6303) infection in young (2 months) and aged (19 months) adult male and female BALB/c mice. (B) Clinical scores (0hr: P=0.0005, 24hr: P=0.0013, and 48hr: P=0.0073), (C) weight changed (P<0.0001), and (D) survival (P=0.0004) in young and aged mice in response to secondary S. pneumoniae infection. (E) Bacterial titer in lung was assessed in lung homogenates collected at 24 hours post infection (primary S. pneumoniae (PBS + S. pne): P=0.0028, secondary S. pneumoniae (INF + S. pne): P=0.0001, young primary vs. secondary S. pneumoniae: P=0.0461, and aged primary vs. secondary S. pneumoniae: P=0.0006). Similar results were obtained from at least two or more independent experiments with an N=5 per experiment. Data are expressed as the mean ± SEM.
