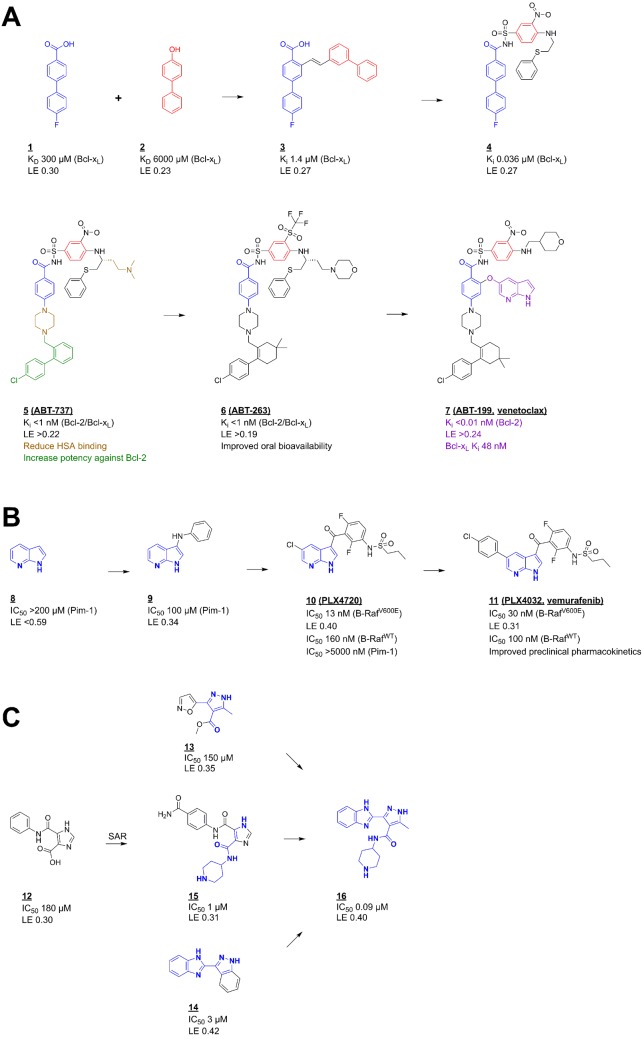
Figure 4. Fragment to lead (to drug) optimization.
(A) Fragment linking – the discovery of venetoclax – a selective Bcl-2 inhibitor; two-site screening by protein observed NMR followed by structure determination and optimization identified fragments ‘1’ and ‘2’ as inhibitors of Bcl-xL. The initial combination of these to ‘3’ and then ‘4’ gave a potent inhibitor that was subsequently optimized to ‘5’ that as ABT-737 entered clinical trials as a dual Bcl-xL/Bcl-2 inhibitor [41] that established proof of concept that inhibition of these anti-apoptotic proteins generates a therapeutic effect. Subsequent design of ‘6’ (ABT-263) gave a compound with improved properties that highlighted that Bcl-xL inhibition led to thrombocytopenia, further design led to the selective Bcl-2 inhibitor ‘7’ that as venetoclax was approved for chronic lymphocytic leukaemia (CLL) [6]. (B) Fragment growing – the discovery of vemurafenib – a selective inhibitor of the B-Raf V600E mutant kinase; fragment ‘8’ was identified from a biochemical screen against the kinase Pim-1 and early structure-guided design used structures bound to Pim-1 and FGFR to identify fragment ‘9’ and the compound ‘10’ (PLX4720). This was then optimized to ‘11’ (vemurafenib) that is now used for the treatment of mutant B-Raf driven melanoma [7]. (C) Fragment merging – a ligand-observed NMR screen against the protein kinase, PDPK1, identified fragments ‘12’ and ‘13’ and compound ‘14’ was identified by SAR by catalogue and subsequent optimization. All these information were combined with the core of the promiscuous literature inhibitor ‘15’ to give the optimized compound ‘16’ that was relatively selective over other kinases, active on cells and gave the expected pharmacodynamics marker responses when administered in vivo [42].