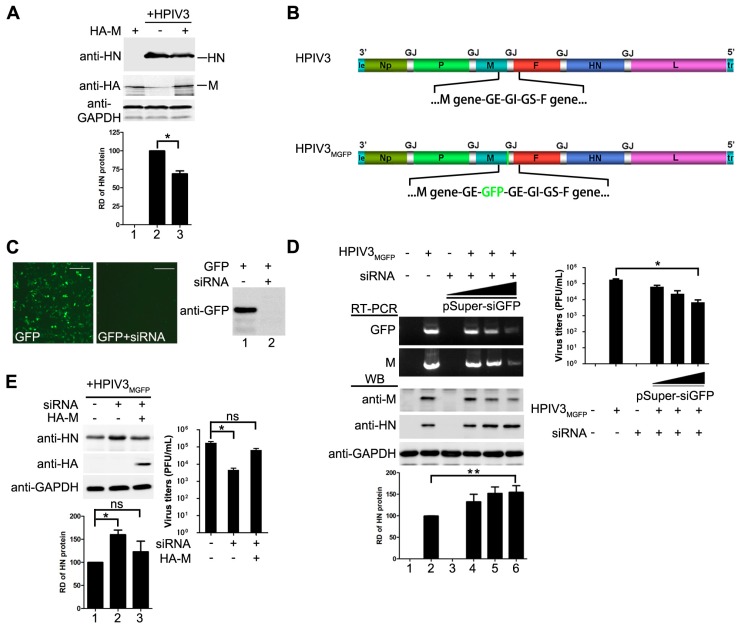
Figure 2.
M reduces HPIV3 replication. (A) over-expression of M reduces the replication of HPIV3. HeLa cells were transfected with vector or plasmids encoding M (2 µg). At 24 hpt, the cells were infected with HPIV3. At 48 hpt, the cells were harvested and lysed for Western blot. Quantity one software was used to quantify the band intensities of HN in the lysates. Values are means ± standard deviations (SD) from three independent experiments. Student’s t test: * p < 0.05; “+,−” means with or without transfecting M plasmid. (B) genomic structure of HPIV3GFP generated in this study. A GFP gene was inserted into the gene end of M and the recombinant virus was rescued as described in Materials and Methods; (C) validity test of pSuper-siGFP. Plasmid encoding GFP (50 ng) was transfected alone or together with 2 µg of pSuper-siGFP in HeLa cells. At 24 hpt, the cells were subjected to fluorescence detection and Western blot assay;“+,−” means with or without transfecting corresponding plasmids. Scale bar, 100 µm. (D) knocking down of M slightly promotes the replication of HPIV3 and extensively reduces viral budding. HeLa cells were transfected with increasing amounts of pSuper-siGFP (0.5 µg, 1 µg, 2 µg). At 24 hpt, the cells were infected with HPIV3MGFP. At 48 hpi, half of the cells were collected for RNA extraction. RT-PCR was used to confirm the effect of siGFP on the transcription of M-GFP mRNA. The other half of cells was lysed for Western blot to detect the expression of M and HN. Supernatants were harvested for titer determination. Quantity one software was used to quantify the band intensities of HN in the lysates. Values are means ± standard deviations (SD) from three independent experiments. Student’s t test: * p < 0.05, ** p < 0.01; “+” means positive transfected or infected corresponding plasmids or viruses. “−” means negative transfected or infected corresponding plasmids or viruses. (E) re-expression of M rescues viral titer and counteracts effect of pSuper-siGFP knockdown. HeLa cells were transfected with 2 µg of pSuper-siGFP. At 24 hpt, the cells were infected with HPIV3MGFP. At 24 hpi, HeLa cells were transfected with 1 µg of pcDNA3.0 vector or pcDNA3.0-HA-M. At 48 hpi, cells were harvested and lysed for Western blot assay. Supernatants were harvested for titer determination. Quantity one software was used to quantify the band intensities of HN in the lysates. Values are means ± standard deviations (SD) from three independent experiments. Student’s t test: * p < 0.05, ns = not significant. “+,−” means with or without transfecting corresponding plasmids.