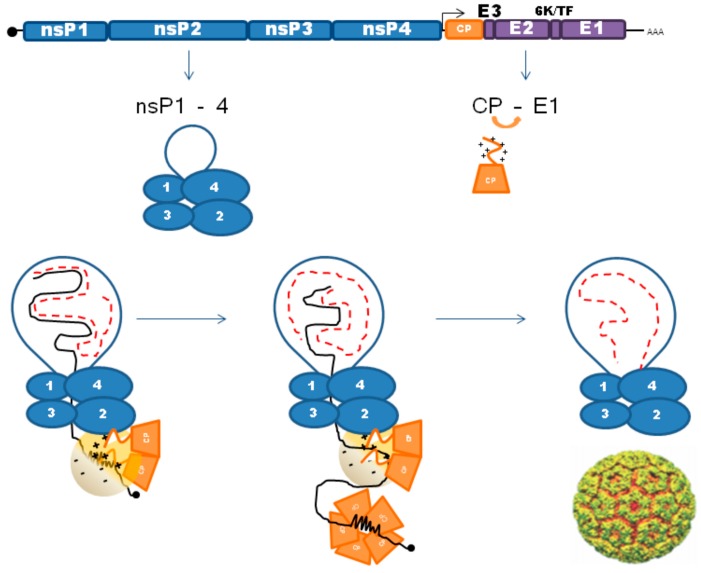
Figure 2.
Model of NC assembly: Upon entry into the cell the alphavirus genome RNA is initially translated into the replication proteins nsP1-4. A spherule results when the replication complex of nsP1–4 (1–4) begins to synthesize RNA. RNA synthesis utilizes a negative strand (red dashed line) intermediate to make more genomic RNA (solid black line). As the replication complex matures, the subgenomic promoter is favored resulting in the structural polyprotein CP to E1. The protease activity of CP on its C-terminal domain cleaves it off nascent polyproteins co-translationally. Evidence thus far suggests that dimeric CP initiates assembly via multiple interactions with its positively charged N-terminal domain and the newly synthesized RNA. This process may be mediated by specific interactions between SD4 and the PS on the genome and a potential interaction between CP and the replication complex (nsP2). Assembly beyond this point is largely a mystery as there is yet to be any evidence of intermediate states. Comparison to the multiple packaging signal hypothesis of Stockley and colleagues suggests that electrostatics play a comparatively more prominent role in alphavirus assembly. However, this does not rule out the theory that RNA compaction could be involved. NC cores accumulate in the cytoplasm and can be visualized as discrete entities at sites distal to replication spherules.