Figure 3.
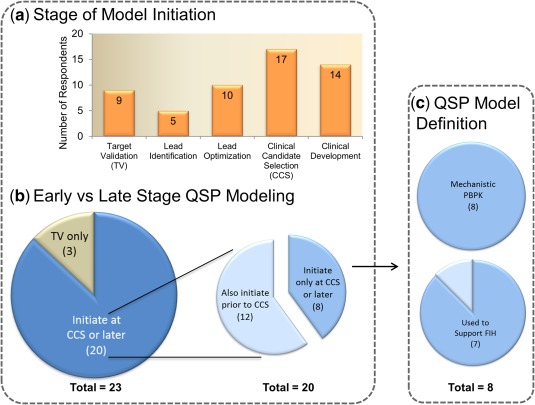
Quantitative systems pharmacology (QSP) model initiation by stage of drug discovery/development. (a) The QSP models are being implemented across all stages of drug discovery/development. (b) Three companies are implementing QSP modeling at the target validation stage only, whereas 20 initiate at clinical candidate selection (CCS) or later. Of those 20, 60% also have activities prior to CCS. Those who only initiated QSP at CCS or later were examined further to understand the role of PBPK and FIH support. (c) In these cases, all considered mechanistic physiologically based pharmacokinetic (PBPK) as a type of QSP model, suggesting that QSP may be used in clinical dose prediction or related pharmacokinetic questions. This hypothesis may be strengthened by the fact that seven of the eight responders also sited that QSP modeling was used to support first‐in‐human (FIH) studies.
