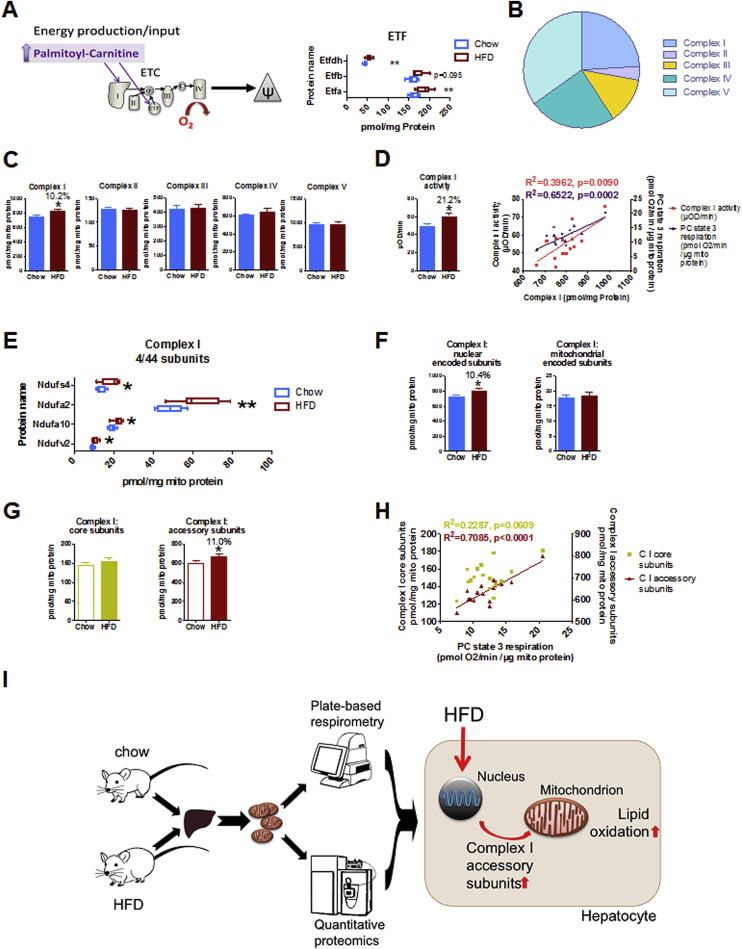
Figure 4.
HFD-induced lipid oxidation is facilitated by increased complex I content and activity, regulated by nuclear accessory subunits. (A) Scheme showing palmitoyl-carnitine entry into the electron transport chain (ETC). Electron transfer flavoprotein (ETF) subunits are increased in HFD-liver mitochondria. (B) The pie chart shows relative proportion of each complex of the ETC. Single subunit concentrations were summed up for each respiratory complex. (C) Total cumulative concentration of respective ETC complex subunits, depicted as group mean values. Asterisk indicates significantly increased complex I concentration in HFD. (D) Respiratory complex I activity by colorimetric assay (bar chart). Cumulated complex I concentrations correlate significantly with complex I activity (red) and PC-state 3 respiration (purple). (E) The absolute concentrations of complex I subunits that are significantly changed by HFD (see Fig. S5 for the complete list of absolute subunit concentrations). (F) The additive concentration of nuclear-encoded complex I subunits (left panel) is increased by HFD, while mitochondrial-encoded subunits are not (right panel). (G) The cumulative concentrations of complex I core and accessory subunits were correlated with (H) PC-state 3 respiration. (I) The integrative analysis of respirometry and proteomics supports model of bioenergetic adaptation in response to HFD that permits increasing lipid oxidation by upregulation of nuclear-encoded accessory complex I subunits. All data represent n = 8 animals. Boxplots indicate 25–75 percentiles, with the vertical line indicating the median and whiskers from minimum to maximum (A, E) or as mean ± standard error of mean. *p < 0.05, **p < 0.01 comparing chow vs HFD by t-test (A, C, D, E, F, G). (D, H) R2 and p-value of Pearson-correlation are given, linear regression is shown where Pearson-correlation is significant (p < 0.05).