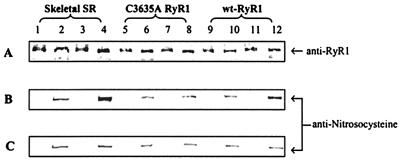
Figure 2.
Immunoblots of nitrosocysteine. Skeletal SR vesicles (lanes 1–4, 10 μg each lane) and cell membrane fractions containing C3635A RyR1 (lanes 5–8, 40 μg each lane) or wt-RyR1 (lanes 9–12, 40 μg each lane) were incubated for 1 hr at 24°C in pO2 ≈ 10 mmHg or pO2 ≈ 150 mmHg in the presence of 1 μM NO. Anti-nitrosocysteine negative controls (treated with 0.1 mmHgCl2, odd-numbered lanes), samples without NO (lanes 2, 6, and 10), and samples treated with 1 μM NO (lanes 3 and 4, 7 and 8, 11 and 12) at pO2 ≈ 10 mmHg (B) and pO2 ≈ 150 mmHg (C) were separated by SDS/3–12% PAGE gradient. The proteins were transferred to Immobilon-P membranes overnight at 4°C. A polyclonal anti-nitrosocysteine antibody was used to detect an S-nitrosylation signal in the protein band region of RyR1 probed with D110 monoclonal anti-RyR1 (A). The data are representative of three experiments.