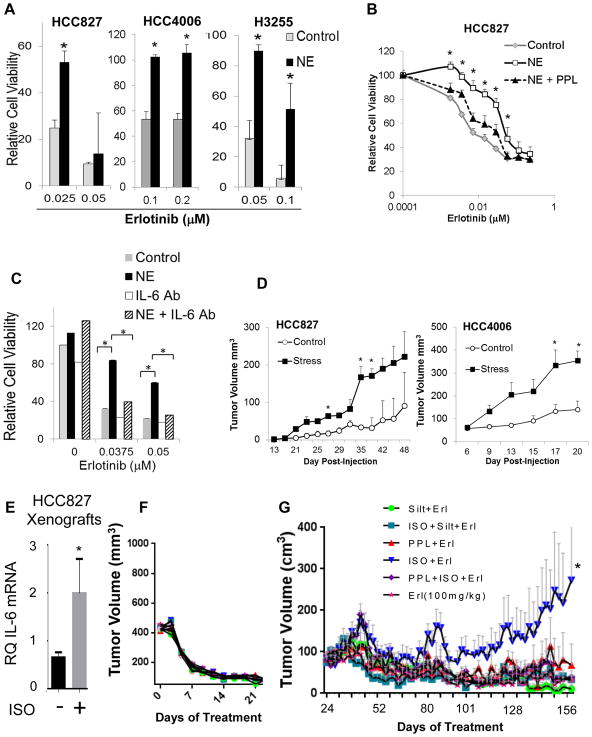
Fig. 4. Stress hormones promote EGFR TKI resistance in vitro and in vivo.
(A) EGFR mutant NSCLC cells were stimulated with NE (10 μM) for 24 hours and then treated with erlotinib. Cell viability was determined by MTS assay. *p<0.0001; by two-tailed Student’s t-test. Bars are means ± s.d. (B) HCC827 cells were treated with propranolol (PPL; *p≤0.03) or (C) IL-6 neutralizing antibodies (*p<0.001) before NE stimulation. After 24 hours, cells were treated with erlotinib for 5 days. Cell viability was evaluated by MTS assay. (D) Ten mice per cell line were injected subcutaneously (control n=5; stress n=5). Chronic stress was induced via restraint. For HCC4006, all mice developed tumors. For HCC827, 4 and 2 mice developed tumors in the stress and control groups, respectively. Data are graphed as means ± s.e.m. *p<0.04; two-tailed Student’s t-test. (E) HCC827 tumor-bearing mice were treated with isoproterenol (β-AR agonist) for three days. Tumors were collected, and IL-6 mRNA was measured by quantitative PCR (control group n=13; ISO group n=7, run as technical duplicates). *p=0.02 two-tailed Student’s t-test. Data are graphed as means ± s.e.m. (F) Erlotinib induced tumor regression in HCC827 xenografts. (G) After prolonged erlotinib treatment, resistant disease emerged in mice treated with erlotinib plus isoproterenol compared to mice receiving erlotinib alone (p=0.018). PPL (p=0.035) or IL-6 antibody, siltuximab (Silt, p=0.009), blocked the effect. *p=0.018; means ± s.e.m.