Fig. 7. Clinical assessment of efficacy of subconjunctival D-Dex and Free Dex gel for corneal opacity and neovascularization.
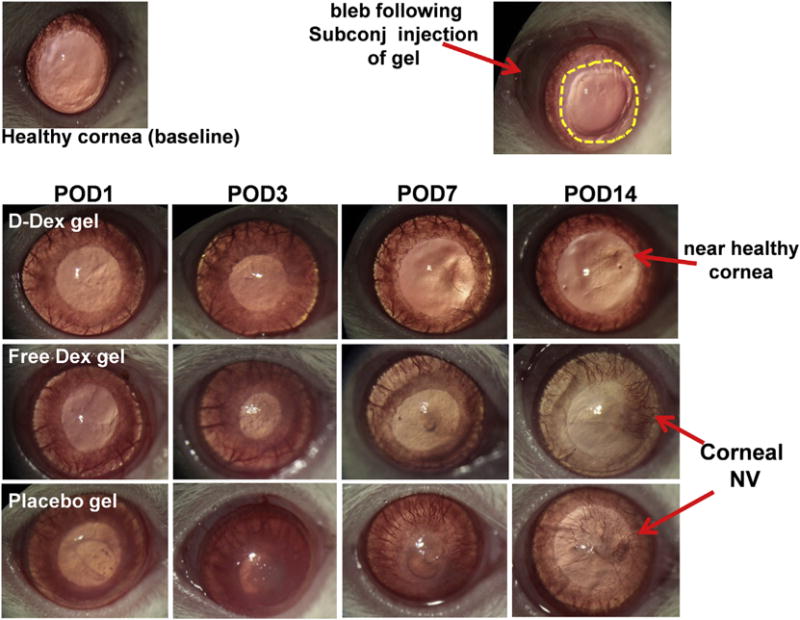
Top left: Photograph of a healthy control: a regular iris and a clear cornea without neovascularization are depicted. Top right: Photograph of a rat cornea immediately after alkali burn demonstrates an epithelial defect (yellow dotted lines); a bleb (red arrow) resulted from the subconjunctival injection of D-Dex gel. Top Panel: Photographic images of a D-Dex gel treated eye over time. The images show a gradual decrease in corneal opacity and minimal corneal neovascularization. Middle panel: Free Dex gel treated group: The eye did not recover over a 14-day period with residual corneal opacity, an irregular corneal light reflex suggesting irregular epithelium and development of clinically significant corneal neovascularization. Bottom panel: A placebo gel treated eye shows extensive damage with a persistent epithelial defect until POD7, corneal opacity and 360° of neovascularization reaching the center of the cornea. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
