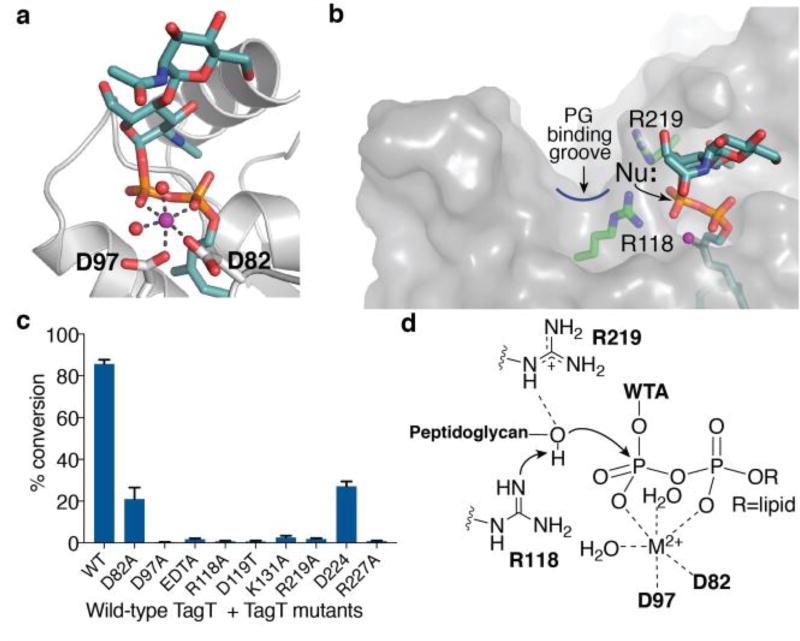
Figure 3.
Crystal structure of TagT and LIIAWTA defines the LCP active site for WTA transfer. a) A close-up view of the LIIAWTA substrate showing the pyrophosphate coordinated by Mg2+ (purple sphere), two waters (red spheres) and aspartate oxygen atoms of D82 and D97. b) In-line attack of the nucleophile on the pyrophosphate defines the location of the PG nucleophile. c) Mutations of conserved charged residues in the active site reduce or abolish TagT activity. All points are the mean ± s.e.m. (n=3). d) Proposed mechanism for deprotonation of the nucleophile.