Table 1.
Enantioselective dioxytosylation of styrenes 1 using aryl-λ3-iodanes 4.a
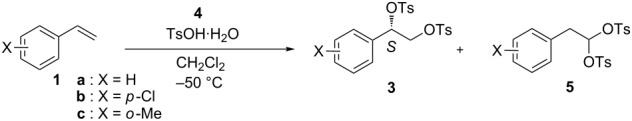 | |||||
| Yield (%)b | |||||
| Entry | Substrate | Reagent | 3 | 5 | ee of 3 (%)c,d |
| 1 | 1a (X = H) | 4a | 53 | 15 | 70 (S) |
| 2 | 1a (X = H) | 4b | 49 | 16 | 80 (S) |
| 3 | 1a (X = H) | 4c | 41 | 14 | 78 (S) |
| 4 | 1a (X = H) | 4d | 41 | 22 | 70 (S) |
| 5 | 1a (X = H) | 4e | 80 | 20 | 92 (S) |
| 6 | 1b (X = p-Cl)e | 4a | 63 | 6 | 70 |
| 7 | 1b (X = p-Cl)e | 4b | 46 | 5 | 76 |
| 8 | 1b (X = p-Cl)e | 4e | 79 | 5 | 90 |
| 9 | 1c (X = o-Me) | 4a | 7 | 34 | 79 |
| 10 | 1c (X = o-Me) | 4e | 10 | 35 | 96 |
aThe reaction was carried out at −50 °C in dichloromethane containing 4 (47 mM), TsOH (86 mM), and 1 (43 mM) for 4 h. bThe yield was determined by 1H NMR using an internal standard. cThe ee was determined by chiral HPLC using a Daicel CHIRALPAK AD column (ø 4.6 mm × 250 mm). dPreferential configuration of product 3. The absolute stereochemistry of 3b and 3c was not determined. eThe reaction was carried out for 20 h.
