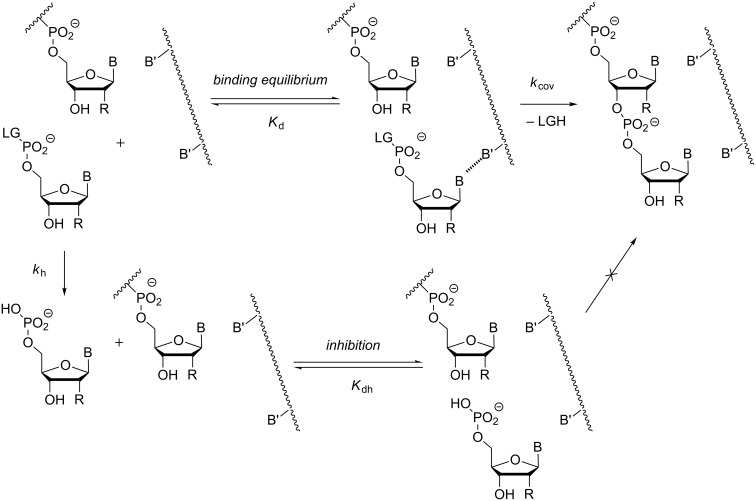
Figure 7.
Steps and equilibria considered in our quantitative model of chemical primer extension [34]. The model considers the binding of the activated monomer with its leaving group (LG) to the primer–template complex in the form of the dissociation constant (Kd). It takes into account the rate of hydrolysis with the corresponding rate constant (kh), the binding equilibrium for the hydrolyzed monomer that acts as inhibitor (Kdh), and it assumes a single rate-limiting chemical step (kcov); B, B' = nucleobase = OH for RNA.