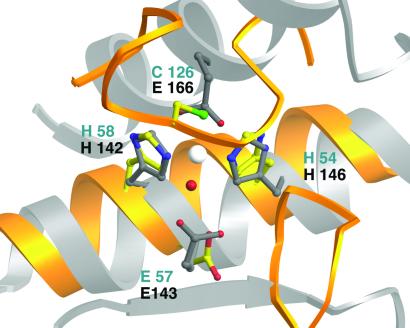
Figure 4.
Comparison of the LuxS zinc-binding site with thermolysin. A molecular superposition based on the metal-ligand clusters of thermolysin (52) and LuxS shows the resulting positions of secondary structure elements from the two enzymes. The backbone ribbons of LuxS are yellow; ribbons from thermolysin are silver. The Zn ligands of LuxS are in standard atom colors; residues from LuxS are labeled in blue, and those from thermolysin, in gray. Helices carrying the HEXXH and HXXEH motifs run in opposite directions but correspond well in the two structures, matching the catalytic glutamate of thermolysin with the conserved Glu-57 from LuxS. The other substructures of the proteins are not equivalent. In thermolysin a long horizontal groove, delimited by strands at its lower edge and by two helices at its upper edge, is accessible to polypeptide substrates. In LuxS the substrate cavity is closed off by loops and by sheet strands from both chains (Figs. 2 and 3).