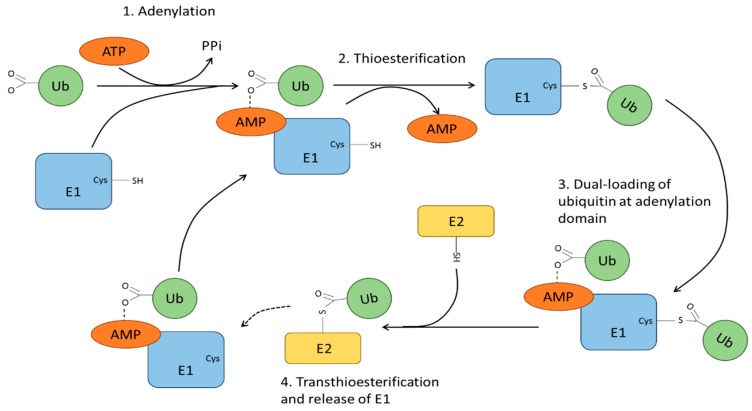
Figure 4.
Ubiquitin activation and conjugating by E1 and E2 enzymes. The E1 enzyme binds to either ubiquitin or ubiquitin-like modifier and Mg-ATP, resulting in ATP hydrolysis. This allows the adenylation of the ubiquitin/ubiquitin-like modifier and release of pyrophosphate. This is followed by the formation of a thioester bond between a cysteine residue within the E1 and the C-terminal glycine of the ubiquitin molecule accompanied by release of AMP. The E1 subsequently undergoes a second round of ubiquitin loading at the adenylation site prior to interaction with the E2. The transfer of the ubiquitin from E1 to a cysteine residue on the E2 occurs via transthioesterification, allowing the release of the E1.