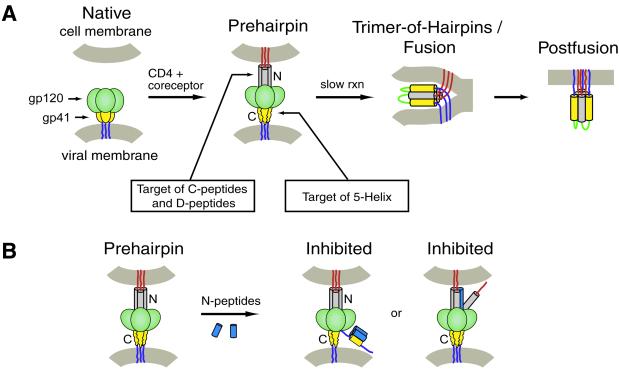
Figure 1.
(A) Working model of HIV-1 membrane fusion and its inhibition (for review, see ref. 1). Before exposure to cellular receptors, Env exists in a native state (“Native”) on the surface of the virus. After interaction with CD4 and the coreceptor, a conformational change allows gp41 to insert its amino-terminal fusion-peptide domain into the cell membrane, forming a transient prehairpin intermediate (“Prehairpin”). In the prehairpin intermediate, the N-peptide region (gray) and possibly the C-peptide region (yellow) are exposed and vulnerable to inhibitors [e.g., C peptides (3, 6, 7, 9, 10), D peptides (18), and 5-Helix (21)]. Although 5-Helix is depicted as targeting the prehairpin intermediate, it is unknown whether it targets the native state, the prehairpin intermediate, or both (see ref. 1). All of these inhibitors work in a dominant-negative manner by preventing formation of the trimer-of-hairpins (see text). In the absence of inhibitors, the prehairpin intermediate slowly resolves to the trimer-of-hairpins structure that juxtaposes the virus and cell membranes and leads to fusion. (B) Representation of two possible mechanisms for N-peptide inhibitory activity. N peptides may target a vulnerable C-helix region of gp41 (yellow). Alternatively, the N peptides (blue) could intercalate with the N helices of gp41 (gray) to form a heterotrimeric coiled coil and interfere with the coiled-coil formation of gp41.