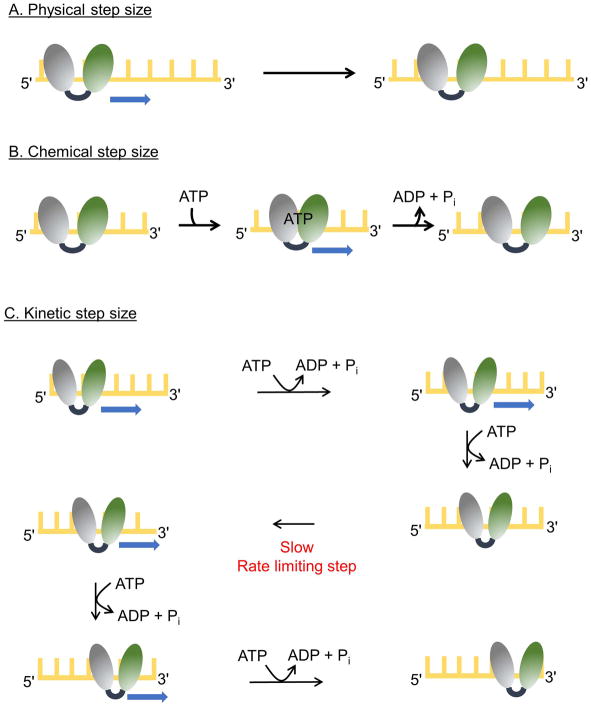
Figure 3. Step sizes of for helicases.
The two RecA-like domains of a helicase are shown (gray and green) and the yellow lattice represents DNA. (A) A helicase with a physical step size of one nucleotide is depicted. The enzyme moves by one nucleotide in each step. (B) A helicase with chemical and physical step sizes of one nucleotide is shown. Binding of ATP results in movement of the rear domain forward by one nucleotide closing the cleft between the two RecA-like domains. Release of ADP and Pi results in movement of the leading domain forward by one nucleotide as the cleft opens. (C) The scheme illustrates movement by a helicase with physical and chemical step sizes of one nucleotide and a kinetic step size of 2 nucleotides. The enzyme moves in single nucleotide steps, each powered by the hydrolysis of a single ATP molecule. After two forward steps, a slow kinetic step occurs which limits the overall rate, resulting in an observed step size of 2 nucleotides. After the slow kinetic step, ATP hydrolysis and forward movement resume.