Figure 1. Coordinated responses to mitochondrial stress.
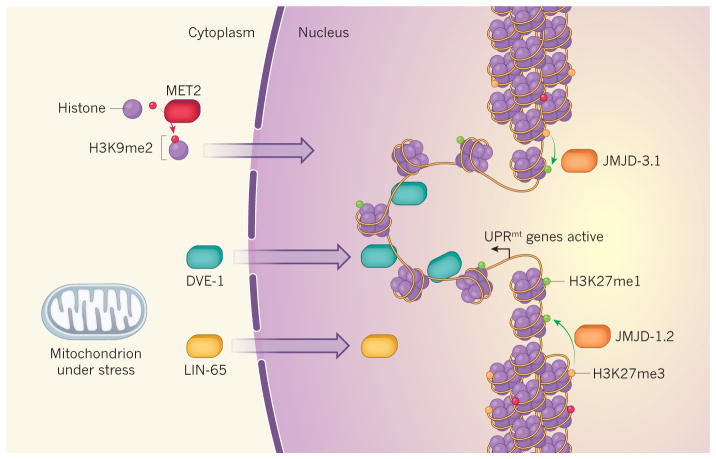
Gene expression can be inhibited by the tight packaging of DNA as chromatin around histone proteins that are decorated with methyl groups at specific amino-acid residues. Two such repressive modifications are dubbed H3K9me2 and H3K27me3. Two studies5,6 reveal that mitochondrial stress triggers the unpackaging and expression of genes involved in a stress response called the UPRmt. Tian et al.5 find that movement of the protein LIN-65 from the cytoplasm to the nucleus triggers nuclear migration of the transcription factor DVE-1. The enzyme MET2 mediates the addition of H3K9me2 modifications to histones in the cytoplasm; these histones move to the nucleus, restricting the unpackaged regions where DVE-1 can bind to promote transcription. Merkwirth et al.6 show that JMJD-1.2 and JMJD-3.1 enzymes are upregulated in response to mitochondrial stress and remove methyl groups from H3K27me3 to form the transcription-promoting modification H3K27me1 in the vicinity of UPRmt genes.