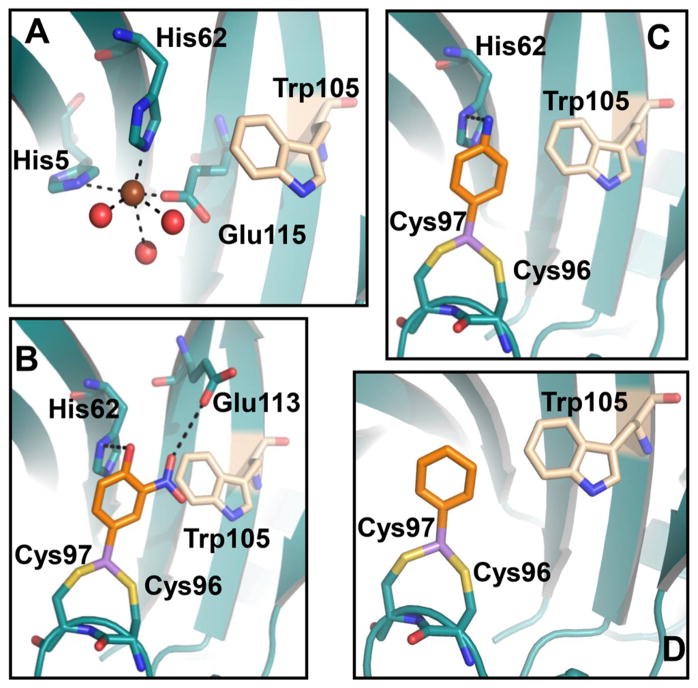
Figure 8.
Model of interaction of Bacillus ArsI with (A) Fe(II), (B) Rox(III), (C) p-ASA(III), and (D) PhAs(III). Fe(II) (brown sphere) directly coordinates with protein through residues His5, His62, and Glu115 and water molecules (red sphere) (A). The aromatic ring of Rox(III) stacks with the five-membered ring of His62 and the hydrophobic side chain of Trp105. The hydroxyl and nitro groups make contact with His62 and Glu113, respectively (B). p-ASA(III) interacts in a similar manner but has no contact with Glu113 (C). PhAs(III) has no contact with either Glu113 or His62 (D). Dotted lines represent hydrogen bonding. Homology modeling was performed as described in Materials and Methods.