Abstract
The effect of ethanolic extract of Morinda citrifolia leaves and fruit on blood pressure in dexamethasone-induced hypertension rat was evaluated. Total phenolic content of Morinda citrifolia leaves ethanolic extract (MCLEE) and Morinda citrifolia leaves ethanolic extract (MCFEE) was 1.789 ± 0.116 and 1.677 ± 0.051 mg of gallic acid equivalents per gram sample, respectively. Rutin level in MCLEE was 0.92 ± 0.19%, and scopoletin level in MCFEE was 0.46 ± 0.05%. MCLEE, MCFEE, and its extract combination significantly decreased the blood pressure of hypertensive rats. The combination group showed highest hypotensive activity by lowering systolic blood pressure by 16.71 ± 3.95%, diastolic blood pressure by 21.49 ± 7.90%, and mean arterial blood pressure by 19.58% ± 6.35. All extract treatments have not been able to repair or inhibit renal damage caused by dexamethasone induction.
Keywords: Morinda citrifolia L, rutin, scopoletin, hypotensive, dexamethasone, renal rat
Hypertension is a major contributor to blood vessels–related diseases such as stroke, myocardial infarction, chronic renal failure, and congestive heart failure.1 Dexamethasone is a steroid hormone produced by the adrenal glands that has activity as glucocorticoid and as precursor of aldosterone. It can cause hypertension due to lower levels of antioxidants and decrease the level of nitric oxide (NO) in the body.2,3 Increased production of reactive oxygen species (ROS) as a result of oxidative stress could trigger the formation of lipid peroxides that are toxic and cause cell damage such as in renal tubular cells and glomerolus.4,5
Morinda citrifolia L is a plant widely used as a herbal medicine for various diseases, that is, used to treat antidyslipidemia, used as an antioxidant, inhibits the activity of angiotensin converting enzyme (ACE), used as an analgesic, and used to treat hypoglycemia.6–9 Reportedly, ripe Morinda citrifolia fruit has antihypertensive activity by inhibiting ACE.6 Morinda citrifolia leaves contain many flavonoids including rutin, which can lower blood pressure by increasing the activity of glutathione peroxidase and NO in the endothelial cells, causing vasorelaxation of blood vessels.10 Both leaves and fruit of Morinda citrifolia have been reported for hypotensive activity, but simultaneous development in the use of fruit and leaves has yet been reported. In the present study, we investigated the hypotensive effect of the combination of ethanolic extracts of Morinda citrifolia leaves and fruit.
Materials and Methods
Materials
Captopril and dexamethasone were obtained from PT Kimia Farma (Jakarta, Indonesia). Aquadest and ethanol 96% were purchased from Bratachem (Jogjakarta, Indonesia). The phytochemical study used rutin, scopoletin (Sigma Chemical Co, St Louis, MO), gallic acid, Folin-Ciocalteau reagent, sodium carbonate, methanol, toluene, n-butanol, ethyl acetate, glacial acetic acid, and formic acid (E. Merck, Darmstadt, Germany).
Animals
Male Wistar rats weighing 150 to 200 g (2-3 months old) used in the study were maintained under standard laboratory condition in a 12:12 h light-dark cycle (light on at 07:00 am) at constant temperature (22 ± 2°C) and relative humidity (55 ± 10%). All animals were fed with a standard laboratory food and water at libitum. The experiment procedure was performed according to ethical clearance from Research Ethics Committee, Integrated Research and Testing Laboratory, Universitas Gadjah Mada, Indonesia (No. 266/KEC-LPPT/V/2015).
Preparation of Ethanolic Extract of Morinda citrifolia Leaves and Fruit
Morinda citrifolia leaves and fruit were obtained from Sendangguwo, Semarang, in December 2014, and authentication was done in the Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta (Certificate Number BF/215/Ident/Det/IV/2015). Separately, dried powder of Morinda citrifolia leaves and fruits (1 kg) were extracted by maceration methods using 96% ethanol for 24 hours. The filtrate was mixed after 2 times of re-maceration, and evaporated to obtain a thick extract. From this step, we got 2 extracts: Morinda citrifolia leaves ethanolic extract (MCLEE) and Morinda citrifolia fruit ethanolic extract (MCFEE). Organoleptic test of the extract was conducted using the senses to describe the shape, smell, color, and taste.11
Phytochemical Analysis
MCLEE and MCFEE analyses were performed using thin layer chromatography (TLC), with a stationary phase of silica gel 60 F254. A mobile phase of ethyl acetate-n-butanol-formic acid-aquadest (5:5:2:1 v/v/v/v) was used for MCLEE. Detection of rutin content in MCLEE was performed under UV wavelengths 254 nm and 366 nm compared with standard rutin. Quantitative analysis of rutin was performed by measuring spot intensity in the TLC scanner at a wavelength of 200 to 400 nm. MCFEE analysis was used a mobile phase of toluene-acetic acid (4:0.5 v/v). Detection of scopoletin content in MCFEE was compared with standard scopoletin and performed under UV wavelengths 254 nm and 366 nm. Scopoletin level in MCFEE was calculated by measuring spot intensity in the TLC scanner at a wavelength of 336 nm.
Total Phenolic Content Assay
Total phenolic content in MCLEE and MCFEE was determined by Folin-Ciocalteau method.12 An aliquot (0.5 mL) of extract and standard solution of gallic acid was added in a 10 mL volumetric flask. Five milliliters of Folin-Ciocalteau reagent and 4.0 mL of 1 M Na2CO3 solution were added to the solution and shaken. After 35 minutes of incubation at room temperature, the absorbance against prepared reagent blank solution was measured at 755 nm with an UV-VIS spectrophotometer (Shimadzu 1240). Total phenolic content of extract was expressed as grams of gallic acid equivalents (GAE)/g of extract weight. All samples were analyzed in triplicates.
In Vivo Antihypertensive Activity Test
Rats were divided into 6 groups of 5 animals each, these are, normal, negative control (dexamethasone induction without treatment), positive control (captopril 10 mg/kg body weight [BW]), MCLEE 500 mg/kg BW, MCFEE 500 mg/kg BW, and combination of MCLEE and MCFEE 1:1 (MCLFEE) 500 mg/kg BW. Dexamethasone induction were performed for 14 consecutive days, and on day 7 of treatment captopril and extract were given orally. The parameters of blood pressure included systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MABP), and heart rate measured by Non-Invasive Blood Pressure of CODA on days 0, 7, and 14. Rat body weight was also monitored. On day 14, rat kidneys were dissected and taken for histopathological examination with hematoxylin-eosin staining. Thymus organs were weighed as a marker for glucocorticoids (mg/100 g BW).
Statistical Analysis
Analysis of the results using one-way analysis of variance was carried out to see the difference between treatment groups for each parameter of blood pressure. Renal histopathology was analyzed by descriptive analysis.
Results
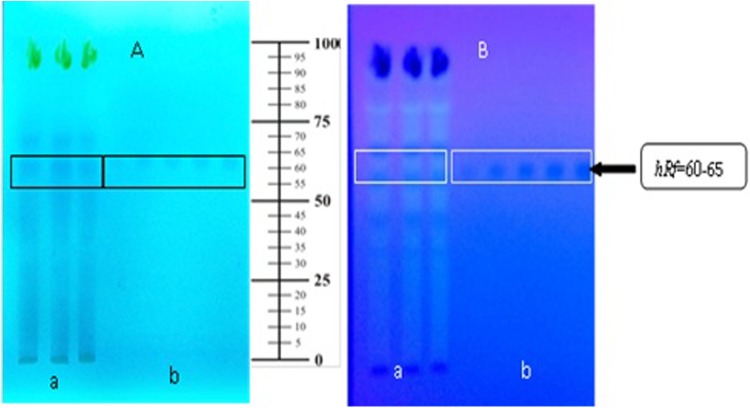
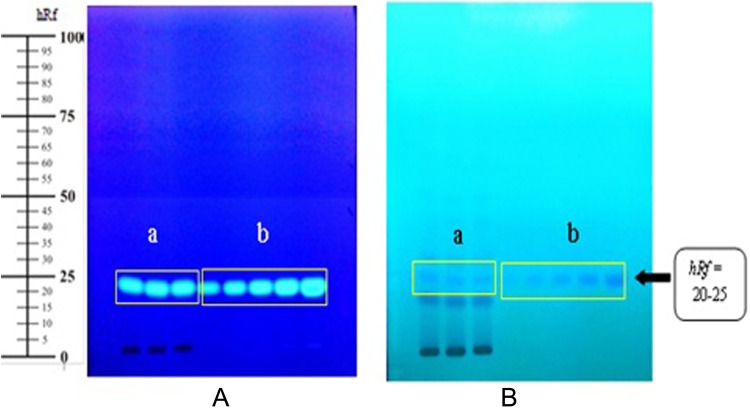
The yield of ethanolic extract of Morinda citrifolia leaves and fruit was 15.74% and 15.78%, respectively. Qualitative identification by TLC and spectral scanning patterns on MCLEE and MCFEE showed phenolic compounds rutin as a marker on MCLEE and scopoletin on MCFEE. The total phenolic content of MCLEE and MCFEE was 1.789 ± 0.116 and 0.051 ± 1.677 mg gallic acid equivalent per g of extract, respectively. Quantitative analysis of marker compounds in the extract was performed using densitometry (Figures 1 and 2). Rutin level in MCLEE was 0.92 ± 0.19%, and scopoletin level in was MCFEE 0.46 ± 0.05%.
Figure 1.
TLC profile of Morinda citrifolia leaves’ ethanolic extract. TLC method was performed using a stationary phase of silica gel 60 F254 and a mobile phase of ethyl acetic-n-butanol-formic acid-water (5:5:2:1 v/v/v/v). (a) Morinda citrifolia leaves ethanolic extract, (b) standard rutin. Spot detection: (A) under UV 254 nm, (B) under UV 366 nm.
Figure 2.
TLC profile of Morinda citrifolia fruit ethanolic extract. TLC method was performed using a stationary phase of silica gel 60 F254 and a mobile phase of toluene-acetic acid (4:0.5 v/v). (a) Morinda citrifolia fruit ethanolic extract, (B) standard scopoletin. Spot detection: (A) under UV 366 nm, (B) under UV 254 nm.
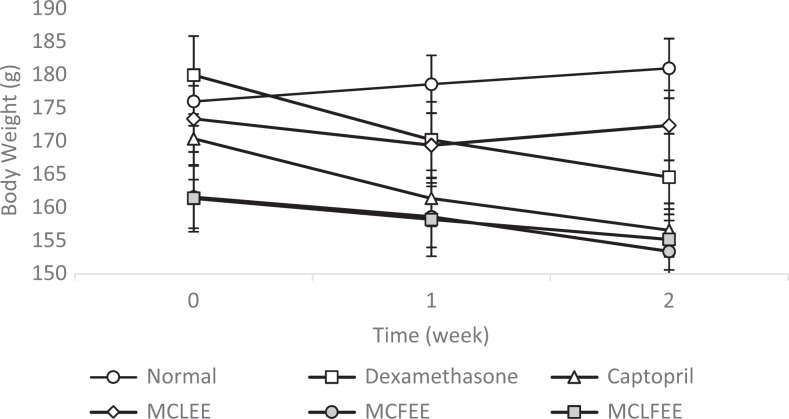
Dexamethasone-induced rats group showed a body weight decrease in comparison to that of the normal group without induction (Figure 3). Decrease in body weight is a metabolic consequence of body weight inhibition growth rate.13 Thymus weight of the normal group was greater than that of the dexamethasone group (Table 1). This is possible due to thymocyte apoptosis and thymus involution.14,15
Figure 3.
Rat body weight after 14 consecutive days of dexamethasone induction. Data presented as mean ± SEM (n = 5).
Table 1.
Thymus Weight Ratio (mg/100 g BW) After 2 Weeks of Treatment.
| No. | Thymus Weight (mg/100 g BW) | |||||
|---|---|---|---|---|---|---|
| Normal | Negative Control | Positive Control | MCLEE | MCFEE | MCLFEE | |
| 1 | 114.6 | 89.2 | 111.3 | 94.6 | 73.0 | 85.6 |
| 2 | 105.0 | 99,8 | 69.8 | 99.9 | 133.9 | 138.5 |
| 3 | 103.8 | 98.9 | 131.1 | 94.3 | 107.6 | 78.0 |
| 4 | 104.4 | 106.7 | 63.3 | 79,0 | 98.4 | 93.3 |
| 5 | 111.0 | 94.7 | 71.8 | 93,9 | 74.8 | 82.8 |
| x ± SD | 107.78 ± 4.8 | 97.85 ± 6.5 | 89.46 ± 29.9 | 92,37 ± 7.9 | 97.56 ± 25.2 | 95.63 ± 24.6 |
Abbreviations: BW, body weight; MCLEE, Morinda citrifolia leaves ethanolic extract; MCFEE, Morinda citrifolia fruit ethanolic extract; MCLFEE, combination of MCLEE and MCFEE (1:1).
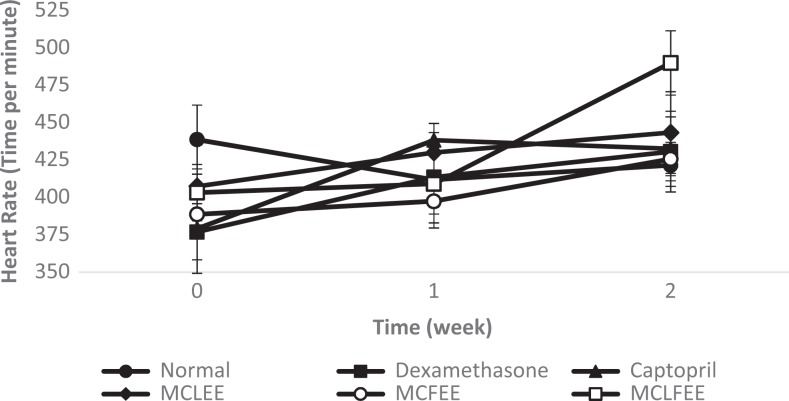
Antihypertensive activity of groups with treatment of extracts either alone or in combination showed a decrease in SBP, DBP, and MABP (Table 2), significantly different in comparison to that of the negative control group (P < .05). The largest percentage in blood pressure decreases was shown in MCLFEE, namely, 16.71 ± 3.95 (SBP), 21.49 ± 7.90 (DBP), and 19.58 ± 6.35 (MABP), which is approaching the percentage decrease in the positive control group given captopril (Figure 4).
Table 2.
Rat Blood Pressure Decrease After 2 Weeks of Treatment.
| Group | Blood Pressure Decrease (%) | ||
|---|---|---|---|
| SBP | DBP | MABP | |
| Captopril | 18.58 ± 1.51 | 18.00 ± 5.33 | 18.31 ± 3.62 |
| MCLFEE | 16.71 ± 3.95 | 21.49 ± 7.90 | 19.58 ± 6.35 |
| MCLEE | 13.73 ± 3.48 | 12.99 ± 3.05 | 13.32 ± 3.12 |
| MCFEE | 11.39 ± 3.07 | 10.57 ± 4.24 | 10.90 ± 3.59 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; MABP, mean arterial blood pressure; MCLEE, Morinda citrifolia leaves ethanolic extract; MCFEE, Morinda citrifolia fruit ethanolic extract; MCLFEE, combination of MCLEE and MCFEE (1:1).
Figure 4.
Rat blood pressure: (a) Systolic, (b) Diastolic, (c) Mean arterial, and (d) Heart rate. Data presented as mean ± SD (n = 5).
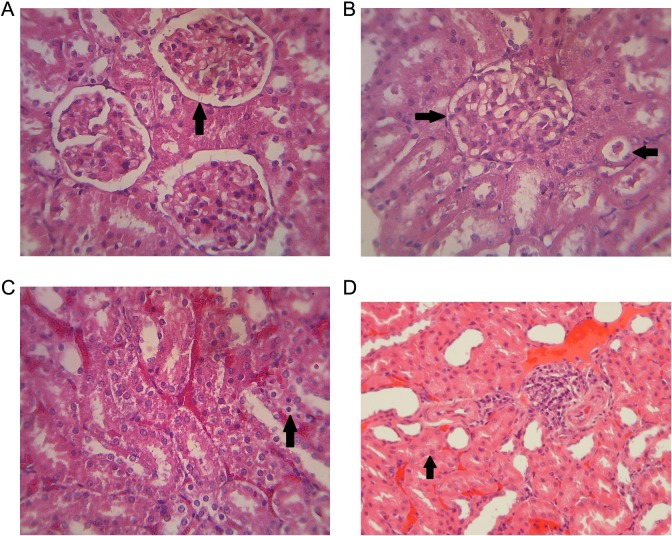
Nitric oxide deficiency and oxidative stress occur on dexamethasone-induced hypertension.16 Oxidative stress would trigger oxidative damage to the kidneys that cause increased levels of malondialdehyde and F2-isoprostanes. ROS effects on mesangial and endothelial cells caused changes on the structure and function of glomerulus.17 Histopathology of kidney rat with hematoxylin and eosin staining in the normal group without treatment did not show any pathological change (Figure 5a). Glomerulus and tubules were still in normal condition. Hydropic degenerations of tubular epithelium, glomerular capillary dilation (Figure 5b), necrosis of epithelial tubules (Figure 5c), and interstitial nephritis (Figure 5d) were observed in the rats induced by dexamethasone of 0.1 mg/kg BW without extract treatment (negative control). It can be concluded that subcutaneous induction of dexamethasone in rats can causes histopathological changes in the kidneys.
Figure 5.
Histopathology of rat renal with hematoxylin-eosin staining. (a) Normal rat, (b-d) Dexamethasone-induced rat. (b) Hydropic degeneration with capillary dilatation, (c) cell necrosis, and (d) nephritis interstitialis. Magnification 10 × 40 eyepiece.
In the group of MCLEE and MCLFEE, treatments for 1 week showed more organ damage than that of the MCFEE-treated group. The negative control group without administration of the extract showed no significant change on animal organs as well as the extract treatment group. However, the control group with captopril administration exhibited kidney improvement. This finding indicates that extract treatment for 1 week was not been able to repair or inhibit damage caused by dexamethasone induction.
Discussion
In the study, SBP, DBP, and MABP of dexamethasone-induced animals were decreased after the treatment of MCLEE, MCFEE, or its combination as well as positive control group. Percentage of blood pressure decrease after treatment of MCLFEE was higher than that of MCLEE and MCFEE, and close to that of the positive control group. MCLFEE is a combination of MCLEE and MCFEE (1:1). Each single extract contains different metabolites that may indicate possible synergistic effects of the combination of the extract. MCLEE contains flavonoid rutin and MCFEE contains scopoletin as indicated by the marker compound. An antihypertensive effect of Morinda citrifolia might be due to rutin and scopoletin.18
Hypertension may develop as a result of increased ROS. Oxidative stress may play a role in the pathophysiology of hypertension by promoting NO deficiency and augmenting arachidonic acid oxidation and formation of vasoconstrictive prostaglandin F2α.19 Ripe fruit and leaf of Morinda citrifolia are high sources of antioxidants.20 Extracts of this plant are able to prevent cell damage from oxidative stress due to their antioxidant activity. Phenolic compounds in plants have the ability to capture free radicals and inhibit lipid peroxidation, so it can prevent cell damage with reduction and electron donor mechanism.21,22 Rutin treatment at dose of 1 g/kg BW intraperitoneally could significantly reduce the level of malonyldialdehyde and improve renal dysfunction by inhibiting ROS through its antioxidant activity.23,24
Flavonoids are compounds that have an important role in the cardiovascular system including blood pressure regulation. Certain flavonoids such as rutin, quercetin, and luteolin showed inhibition effect on the activity of ACE.25 Antihypertensive activity of ripe Morinda citrifolia fruit juice was stronger than that of green ones. This is due to the presence of inhibitors of ACE contained in the ripe Morinda citrifolia fruit juice.6 Reportedly, Morinda citrifolia fruit juice also significantly increased the urine volume of animals.26 Flavanols from tea could inhibit the activity of ACE in the kidneys of mice related to the regulation of the renin angiotensin aldosterone system and increased production of NO.27,28 The polyphenols of Cocos nucifera directly activate NO and stimulate muscarinic receptor via the cyclooxygenase pathway.29
Scopoletin, a coumarin derivative, is one of the most important phenolic compounds in Morinda citrifolia fruit. Scopoletin has hypotension activity by vasodilatation mechanism through its smooth muscle relaxant activity, acting as a nonspecific spasmolytic agent, and might have an ACE inhibitory effect.30,31 Free radical scavenging effect of scopoletin may prevent inactivation of NO by free radicals.32 Reportedly, scopoletin also induced a dose-dependent decrease in inotropic activity plus an appreciable decrease in chronotropic effects, especially at higher dose levels.33
The development of herbal medicine in Indonesia led to the phytopharmaca in which the efficacy is based on pharmacology, toxicity tests on animals, and clinical trials on humans. A successfully developed phytopharmaca for the treatment of hypertension is Tensigard with one ingredient is Centella asiatica.34,35 Curcumin and its derivatives were also tested for vasodilator activity in rat aorta. Sometimes, several medicinal plants or its parts are combined to obtain a better antihypertensive activity.36 In this study, combination of leaves and fruit of Morinda citrifolia exhibited an improvement in its antihypertensive effect compared to each single extract.
The present results enriched the information on biological activities of medicinal plants originated from Southeast Asia that are widely used by traditional medicine systems. In some Asian countries, main sources of new drugs are mainly from medicinal plants, and only a few are from the synthetic drugs.37–40 The Southeast Asian countries have high biodiversity in the world including medicinal plants. Some of them are being investigated through intensive studies.41–44
Conclusion
MCLEE and MCFEE or its combinations succeeded in decreasing SBP, DBP, and MABP of dexamethasone-induced hypertensive rats. However, it is necessary to conduct further research to determine the mechanism of action even though it has been reported that Morinda citrifolia is able to reduce hypertension through the activity of ACE inhibitor and antioxidant activity of phenolic compounds including scopoletin and rutin that could capture free radicals.
Acknowledgments
The authors would like to thank Septiana Laksmi Ramayani at AKFAR Theresiana Semarang, Indonesia, for preparation of the sample.
Footnotes
Author Contributions: DW contributed to the design of research, animal trials, data analyses, and drafted the article. KA assisted with the experiment and prepared the final version of the article. SDN and AEN participated in the design of research, supervised the experiment, provided mentorship support, and helped in the preparation of the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was conducted with financial support of Lembaga Penelitian dan Pengabdian Kepada Masyarakat, Universitas Gadjah Mada, Indonesia, through “Penelitian Unggulan Perguruan Tinggi” Research Grant No. LPPM-UGM/345/LIT/2014.
Ethical Approval: Ethical approval was obtained from the Research Ethics Committee, Integrated Research and Testing Laboratory, Universitas Gadjah Mada, Indonesia (No. 266/KEC-LPPT/V/2015).
References
- 1. Mantero F, Boscaro M. Glucocorticoid-dependent hypertension. J Steroid Biochem Mol Biol. 1992;43:409–413. [DOI] [PubMed] [Google Scholar]
- 2. Kenning I, Kerandi H, Luehr D, et al. Hypertension diagnosis and treatment. Bloomington, MN: Institute for Clinical Systems Improvement; https://www.icsi.org/_asset/wjqy4g/HTN.pdf. Published 2014. Accessed May 30, 2016. [Google Scholar]
- 3. Iuchi T, Akaike M, Mitsui T, et al. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003;92:81–87. [DOI] [PubMed] [Google Scholar]
- 4. Manning RD, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. [DOI] [PubMed] [Google Scholar]
- 5. Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31(suppl 2):S181–S184. [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi S, Ohnishi J, Sogawa M, et al. Inhibition of angiotensin I converting enzyme by noni (Morinda citrifolia) juice. Nippon Shokuhin Kagaku Kogaku Kaishi. 2002;49:624–627. [Google Scholar]
- 7. Kamiya K, Hamabe W, Harada S, Murakami R, Tokuyama S, Satake T. Chemical constituent of Morinda citrifolia roots exhibit hypoglycemic effects in streptozotozin-induced diabetic mice. Biol Pharm Bull. 2008;31:935–938. [DOI] [PubMed] [Google Scholar]
- 8. Basar S, Uhlenhut K, Högger P, Schöne F, Westendorf J. Analgesic and anti-inflammatory activity of Morinda citrifolia L. (noni). fruits. Phytother Res. 2010;24:38–42. [DOI] [PubMed] [Google Scholar]
- 9. West BJ, Jensen CJ, Palu AK, et al. Toxicity and antioxidant test of Morinda citrifolia (noni) seed extract. Adv J Food Sci Technol. 2011;3:303–307. [Google Scholar]
- 10. Shen Y, Croft KD, Hodgson JM, et al. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem Pharmacol. 2012;84:1036–1044. [DOI] [PubMed] [Google Scholar]
- 11. Depkes RI. Parameter Standar Umum Ekstrak Tumbuhan Obat. Jakarta, Indonesia: Departemen Kesehatan Republik Indonesia; 2000. [Google Scholar]
- 12. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Meth Enzymol. 1999;299:152–178. [Google Scholar]
- 13. Safaeian L, Zabolian H. Antioxidant effects of bovine lactoferrin on dexamethasone-induced hypertension in rat. ISRN Pharmacol. 2014;2014:943523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ong SL, Vohra H, Zhang Y, Sutton M, Whitworth JA. The effect of alpha-lipoic acid on mitochondrial superoxide and glucocorticoid-induced hypertension. Oxid Med Cell Longev. 2013;2013:517045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zavitsanou K, Nguyen V, Greguric I, Chapman J, Ballantyne P, Katsifis A. Detection of apoptotic cell death in the thymus of dexamethasone treated rats using [123I]annexin V and in situ oligonucleotide ligation. J Mol Histol. 2007;38:313–319. [DOI] [PubMed] [Google Scholar]
- 16. Ong SL, Zhang Y, Sutton M, Whitworth JA. Hemodynamics of dexamethasone-induced hypertension in the rat. Hypertens Res. 2009;32:889–894. [DOI] [PubMed] [Google Scholar]
- 17. Bandegi AR, Rashidy-Pour A, Vafaei AA, Ghadrdoost B. Protective effects of Crocus sativus L. extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv Pharm Bull. 2014;4:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pandy V, Narasingam M, Kunasegaran T, Murugan DD, Mohamed Z. Effect of noni (Morinda citrifolia Linn.) fruit and its bioactive principles scopoletin and rutin on rat vas deferens contractility: an ex vivo study. ScientificWorldJournal. 2014;2014:909586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beg M, Sharma V, Akhtar N, Gupta A, Mohd J. Role of antioxidants in hypertension. JIACM. 2011;12:122–127. [Google Scholar]
- 20. Yang J, Gadi R, Thomson T. Antioxidant capacity, total phenols, and ascorbic acid content of noni (Morinda citrifolia) fruits and leaves at various stages of maturity. Micronesica. 2011;41:167–176. [Google Scholar]
- 21. Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–179. [DOI] [PubMed] [Google Scholar]
- 22. Nugroho AE, Malik A, Pramono S. Total phenolic and flavonoid contents, and in vitro antihypertension activity of purified extract of Indonesian cashew leaves (Anacardium occidentale L.). Int Food Res J. 2013;20:299–305. [Google Scholar]
- 23. Korkmaz A, Kolankaya D. Protective effect of rutin on the ischemia/ reperfusion induced damage in rat kidney. J Surg Res. 2010;164:309–315. [DOI] [PubMed] [Google Scholar]
- 24. Sunarwidhi AL, Sudarsono S, Nugroho AE. Hypoglycemic effect of combination of Azadirachta indica A. Juss. and Gynura procumbens (Lour.) Merr. ethanolic extracts standardized by rutin and quercetin in alloxan-induced hyperglycemic rats. Adv Pharm Bull. 2014;4:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guerrero L, Castillo J, Quiñones M, et al. Inhibition of angiotensin-converting enzyme activity by flavonoids: structure-activity relationship studies. PLoS One. 2012;7:e49493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shenoy JP, Pai PG, Shoeb A, Gokul P, Kulkarni A, Kotian MS. An evaluation of diuretic activity of Morinda citrifolia (Linn) (noni) fruit juice in normal rats. Int J Pharm Pharm Sci. 2011;3:119–121. [Google Scholar]
- 27. Actis-Goretta L, Ottaviani JI, Keen CL, Fraga CG. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003;555:597–600. [DOI] [PubMed] [Google Scholar]
- 28. Persson IA, Josefsson M, Persson K, Andersson RG. Tea flavanols inhibit angiotensin-converting enzyme activity and increase nitric oxide production in human endothelial cells. J Pharm Pharmacol. 2006;58:1139–1144. [DOI] [PubMed] [Google Scholar]
- 29. Bankar GR, Nayak PG, Bansal P, et al. Vasorelaxant and antihypertensive effect of Cocos nucifera Linn. endocarp on isolated rat thoracic aorta and DOCA salt-induced hypertensive rats. J Ethnopharmacol. 2011;134:50–54. [DOI] [PubMed] [Google Scholar]
- 30. Ojewole JAO, Adesina SK. Mechanism of the hypotension effect of scopoletin isolated from the fruit of Tetrapleura tetraptera. Planta Med. 1983;49:46–50. [DOI] [PubMed] [Google Scholar]
- 31. Kumar R, Kumar A, Sharma R, Baruwa A. Pharmacological review on natural ACE inhibitors. Der Pharmacia Lettre. 2010;2:273–293. [Google Scholar]
- 32. Kwon EA, Jin SS, Choi MH, et al. Mechanism of relaxation of rat aorta by scopoletin: an active constituent of Artemisia capillaris. Korean J Oriental Physiol Pathol. 2002;16:389–396. [Google Scholar]
- 33. Guantai AN, Addae-Mensah I. Cardiovascular effect of Artemisia afra and its constituents. Pharm Biol. 1999;37:351–356. [Google Scholar]
- 34. Djatmiko M, Suhardjono D, Nugroho AE. Pharmacological and dosage range tests of TensigardÒ as a hypotensive phytopharmaca. Indonesian J Pharm. 2001;12:38–44. [Google Scholar]
- 35. Nugroho AE, Suhardjono D, et al. The vasodilation effects of curcumin and its derivatives on isolated aortic of rats. Indonesian J Pharm. 2008;19:70–77. [Google Scholar]
- 36. Harwoko Pramono S, Nugroho AE. Triterpenoid-rich fraction of Centella asiatica leaves and in vivo antihypertensive activity. Int Food Res J. 2014;21:149–154. [Google Scholar]
- 37. Nugroho AE, Ikawati Z, Sardjiman Maeyama K. Effects of benzylidenecyclopentanone analogues of curcumin on histamine release from mast cells. Biol Pharm Bull. 2009;32:842–849. [DOI] [PubMed] [Google Scholar]
- 38. Nugroho AE, Riyanto S, Sukari MA, Maeyama K. Anti-allergic effects of marmin, a coumarine isolated from Aegle marmelos Correa: in vitro study. Int J Phytomed. 2011;3:84–97. [Google Scholar]
- 39. Nugroho AE, Hermawan A, Putri DD, Novika A, Meiyanto E, Kawaichi M. Combinational effects of hexane insoluble fraction of Ficus septica Burm. F. and doxorubicin chemotherapy on T47D breast cancer cells. Asian Pac J Trop Biomed. 2013;3:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nugroho AE, Riyanto S, Sukari MA, Maeyama K. Effects of aegeline, a main alkaloid of Aegle marmelos Correa leaves, on the histamine release from mast cells. Pak J Pharm Sci. 2011;24:359–367. [PubMed] [Google Scholar]
- 41. Nugroho AE, Ikawati M, Hermawan A Putri DD, Meiyanto E. Cytotoxic effect of ethanolic extract fractions of Indonesian plant Ficus septica Burm. f. on human breast cancer T47D cell lines. Int J Phytomed. 2011;3:216–226. [Google Scholar]
- 42. Nugroho AE, Anas Y, Arsito PN, Wibowo JT, Riyanto S, Sukari MA. Effects of marmin: a compound isolated from Aegle marmelos Correa, on contraction of the guinea pig-isolated trachea. Pak J Pharm Sci. 2011;24:427–433. [PubMed] [Google Scholar]
- 43. Nugroho AE, Rais IR, Setiawan I, et al. Pancreatic effect of andrographolide isolated from Andrographis paniculata (Burm. f.) Nees. Pak J Biol Sci. 2014;17:22–31. [DOI] [PubMed] [Google Scholar]
- 44. Nugroho AE, Kusumaramdani G, Widyaningar A, Anggoro AE, Pramono S. Antidiabetic effect of combinations of n-hexane insoluble fraction of ethanolic extract of Andrographis paniculata with other traditional medicines. Int Food Res J. 2014;21:785–789. [Google Scholar]