Figure 11.
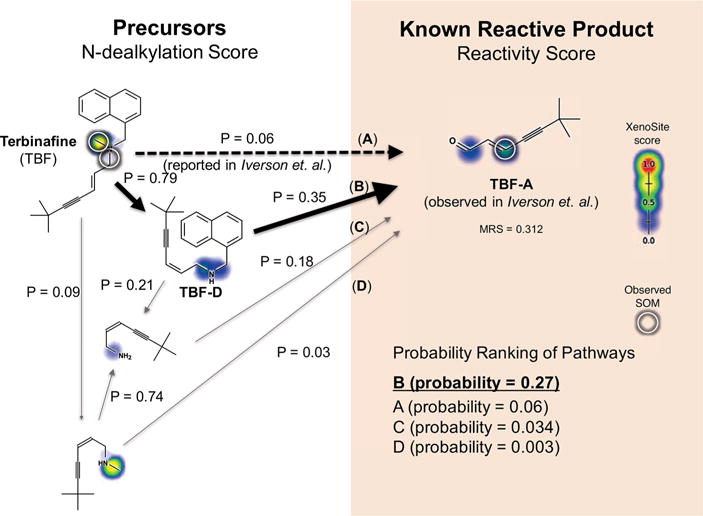
Terbinafine is predicted to form its reactive metabolite by two sequential dealkylations. Multiple metabolites generated by N-dealkylation of terbinafine have been observed.10,54 The reactive metabolite 7,7-dimethylhept-2-ene-4-ynal (TBF-A, depicted) was identified as a key mediator of hepatotoxicity.10 It is proposed that TBF-A is formed directly from terbinafine single dealkylation (dashed arrows, P = 0.06), but several alternate pathways are possible. The model computes the probability of each pathway, finding that TBF-A is most likely formed by two sequential dealkylations (path B, P = 0.27) through the intermediate desmethyl terbinafine (TBF-D). Supporting this prediction, TBF-D is reported as a metabolite of TBF by some studies.54 This suggests a revision of the single dealkylation pathway reported in the literature (path A, P = 0.06).
