Figure 8.
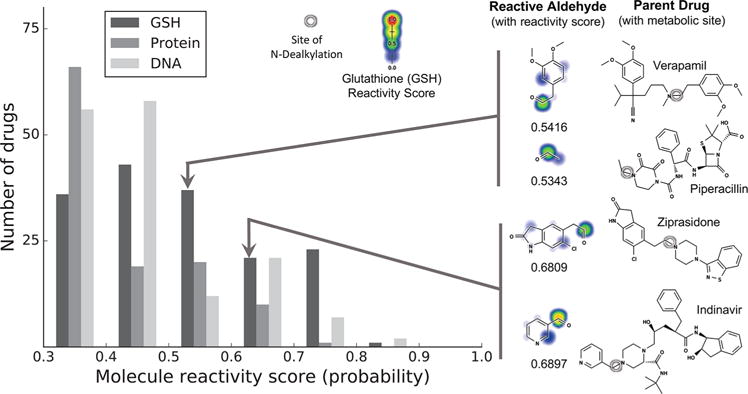
Reactive aldehyde metabolites formed by N-dealkylation reactions. Of 1925 approved and withdrawn drugs, the literature reports N-dealkylation reactions for 380. The aldehydes formed by these N-dealkylation reactions were inferred, and their reactivity was assessed with a previously published reactivity model.5 (Left) The distribution of reactivity scores above 0.3 for glutathione (GSH), protein, and DNA molecule reactivity. For reference, the reactivity scores of N-acetyl-p-benzoquinone imine, a well-known electrophile responsible for acetaminophen’s toxicity, are 0.75, 0.54, and 0.38 for GSH, protein, and DNA, respectively. (Right) Several drugs that are (1) known to be hepatotoxic (2) by unknown mechanisms also (3) appear to form reactive aldehydes. These aldehyde metabolites were predicted to be more reactive than the parent drug as well as their other observed metabolites, so these aldehydes might be the mechanism of toxicity.
