Figure 8.
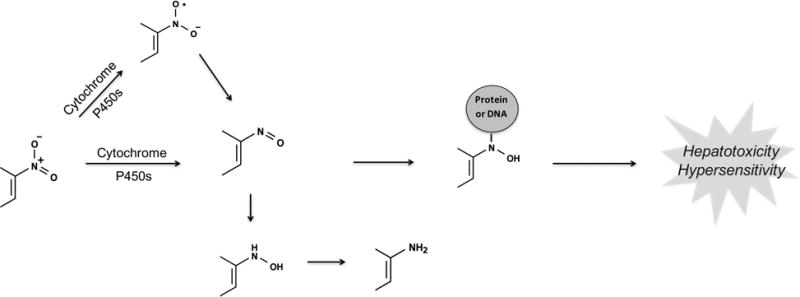
Nitroaromatics are bioactivated through reduction. Nitroaromatic compounds undergo sequential two-electron reductive steps to the nitroso, N-hydroxy, and amine. Alternatively, they can form nitro anion radicals through a one-electron reduction in the absence of oxygen. The reaction chain can also be reversed when an aromatic amine is oxidized to the N-hydroxy/nitroso compound. However, because the intracellular environment is reducing at physiological conditions, the equilibrium usually shifts toward the right.106,107
