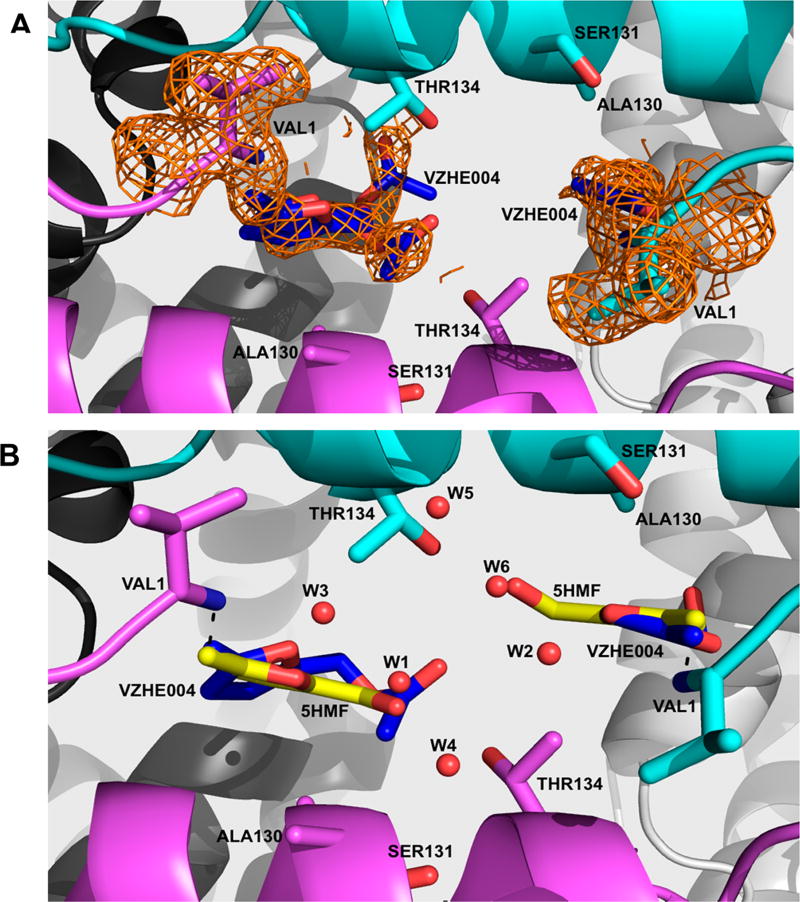
Figure 6.
Crystal structure of carbon monoxide Hb in complex with VZHE004 in the R2-state conformation. Hb subunits are in sticks or ribbons (α1-subunit in cyan, α2-subunit in magenta, β1-subunit in gray, and β2-subunit in white). Water molecules are in red spheres. (a) Pair of VZHE004 molecules (blue sticks) bound at the α-cleft of Hb making Schiff-base interactions with the αVal1N. Superposed on the bound VZHE004 and the N-terminal αVal1 is the final electron-density map with coefficients 2Fo–Fc shown at the 0.6σ level. Note that the compound was refined as 5-HMF at the α1Val1 site (due to apparent disorder at the methyl acetate position), while the full VZHE004 was modeled at the α2Val1 binding site but with the methyl acetate refined in two alternate conformations. (b) VZHE004 (blue sticks) and 5-HMF from the crystal structure 1QXE (bright yellow sticks) superposed on each other showing similar binding modes. Also, shown in Figure S2 is the initial Fo–Fc map (before any ligand was built in the model).