Abstract
Duration of initial disease response remains a strong prognostic factor in multiple myeloma (MM) particularly for upfront autologous hematopoietic cell transplant (AHCT) recipients. We hypothesized that new drug classes and combinations employed prior to AHCT as well as after post-AHCT relapse may have changed the natural history of MM in this population. We analyzed the Center for International Blood and Marrow Transplant Research database to track overall survival (OS) of MM patients receiving single AHCT within 12 months after diagnosis (N=3,256) and relapsing early post-AHCT (<24 months), and to identify factors predicting for early vs. late relapses (24–48 months post-AHCT). Over 3 periods (2001–2004, 2005–2008, 2009–2013), patient characteristics were balanced except for lower proportion of Stage III, higher likelihood of 1 induction therapy with novel triplets and higher rates of planned post-AHCT maintenance over time. The proportion of patients relapsing early was stable over time at 35–38%. Factors reducing risk of early relapse included lower stage, chemosensitivity, transplant after 2008 and post-AHCT maintenance. Shorter post-relapse OS was associated with early relapse, IgA MM, Karnofsky <90, stage III, >1 line of induction and lack of maintenance. Post-AHCT early relapse remains a poor prognostic factor, even though outcomes have improved over time.
INTRODUCTION
Autologous hematopoietic cell transplantation (AHCT) continues to be an integral component of initial treatment strategy in eligible patients with multiple myeloma (MM).1–6 Significant progress has been made in prolonging the duration of initial disease control through judicious combination of effective initial therapy, and AHCT with post-transplant consolidation and maintenance therapy of varying duration.7, 8 However, most patients eventually relapse and the duration of initial disease control appears to be one of the most important prognostic factors for survival in patients with MM, likely a reflection of the underlying high-risk disease biology that may not be always reflected accurately in the baseline laboratory and MM-relevant fluorescent in situ hybridization (FISH) findings.9–11 Prior studies have shown that the time to progression after AHCT reliably predicts the overall survival from the time of relapse and in fact this has been commonly used as a metric for determining the potential benefit from a second AHCT used as salvage therapy.9, 12, 13 In a study of 432 patients transplanted at Mayo Clinic within 12 months of their diagnosis, 94 patients (22%) had relapsed within 12 months of their transplant.12 Median overall survival (OS) from diagnosis was 23.9 months in the early relapse group compared to 82.2 months in the late relapse group. Among the 265 patients who had disease progression after transplant, median overall survival from relapse was only 7.8 months for the early relapse group compared to 39.6 months for the late relapse group. Most of the available data reflect prior treatment approaches and the improvements in therapy over the past decade including the use of new drug classes and routine incorporation of post AHCT maintenance is likely to have altered these estimates. Finally, the risk factors associated with early treatment failure following AHCT as well as those associated with inferior outcomes post-relapse are not well understood in the context of modern therapies, and this knowledge will allow us to better predict risk and design clinical trials to improve outcomes.
We undertook the current study to specifically address how these clinical scenarios and their implications have changed during the recent decade, given the dramatic change in treatments and consequent improvement in OS of patients with MM. Specifically, we wanted to determine if risk of early relapse after AHCT has changed, if OS after early relapse has improved, the factors predicting early and late relapses after AHCT, and to compare post-relapse survival among patients suffering an early relapse (<24 months from transplant) and those with more durable disease control. We used the Center for International Blood and Marrow Transplant Research (CIBMTR) database to conduct this analysis.
PATIENTS AND METHODS
Data Source
The CIBMTR is a prospectively maintained transplant database that captures transplant data from over 500 transplant centers worldwide. Data are submitted to a statistical center at the Medical College of Wisconsin in Milwaukee. Participating centers are required to report all transplants consecutively; patients are followed longitudinally and compliance is monitored by onsite audits. Computerized checks for discrepancies, physicians’ review of submitted data, and onsite audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
The specific objectives for the study were to determine if OS has improved between January 2001 and December 2013 among patients relapsing early (<24 months) after an AHCT, to determine factors predicting early and late relapses (24–48 months) after AHCT and to compare post-relapse survival among early relapse (<24 months from AHCT) and late relapse (24–48 months from AHCT).
Patient Selection
Patients who underwent first AHCT for MM in the United States or Canada from 2001–2013 and reported to CIBMTR were considered for the current study. Patients undergoing late AHCT (>12 months from diagnosis), those undergoing tandem transplants, those receiving non-melphalan based conditioning, and those with unknown induction treatment agents were excluded. Patients were required to have at least 100 days follow up or death prior to 100 days after AHCT and should have consented to research participation; those with unknown relapse status were excluded. The disposition of patients who were considered for inclusion is detailed in supplementary table 1.
Endpoints
The endpoints of interest included disease response, progression-free survival (PFS), and OS after transplant. Disease response was assessed using the International Myeloma Working Group (IMWG) consensus criteria.14 PFS was defined as time without progressive disease with patients alive and without progression/relapse censored at last follow-up. OS was defined as time from diagnosis or time of relapse after AHCT till death from any cause with censoring of surviving patients at last follow-up.
Statistical Methods
We examined the post-transplant OS in the entire cohort from diagnosis of myeloma, and OS from post-AHCT relapse in the group of patients with a documented relapse occurring within 24 months of AHCT comparing it to those with a relapse after 24 months or none at the time of last follow up. Univariate analysis was conducted to compare post-AHCT OS among the early relapse group over time. Patients were divided in 3 groups based on year of transplant, 2001–2004, 2005–2008 and 2009–2013. Two separate multivariate analyses were conducted: 1) Time to relapse from transplant was analyzed to identify factors associated with early relapse and relapse after 24 months. We fitted a left-truncation model where patients who relapsed after 24 months, their relapse time was truncated at 24 months. Factors that were studied included age, gender, race, Karnofsky Performance Score (KPS) at transplant, MM subtype (IgG, IgA, light chain, non-secretory, others), serum creatinine at diagnosis, stage at diagnosis (International Staging System or Durie Salmon Stage III versus I/II), lines of pre-transplant chemotherapy, novel triplet versus novel doublet versus non-novel induction, melphalan conditioning dose, chemosensitivity, disease status at transplant, time from diagnosis to transplant, year of transplant, planned post-transplant therapy; 2) Post-relapse OS- this analysis which was conducted on all relapsed patients and included early versus late relapse in addition to all the aforementioned characteristics in the multivariate model.
RESULTS
The study included 3256 patients who underwent AHCT within 12 months of diagnosis and had data reported to the CIBMTR. The baseline characteristics are as shown in Table 1. The study cohort was divided into three groups based on the year of AHCT: 2001–2004 (n=896), 2005–2008 (n=1401) and 2009–2013 (n=959). Patients in the most recent group were more likely to have received induction therapy with bortezomib, lenalidomide and dexamethasone, compared to the previous years and were more likely to come to transplant with a single line of prior therapy, reflecting the improved efficacy of current regimens. A higher proportion of patients received their transplant within 6 months from diagnosis in the most recent cohort. The median follow up from AHCT of the survivors in the three groups were 120, 86 and 39 months respectively.
Table 1.
Patient characteristics at diagnosis
| Variable | 2001–2004 | 2005–2008 | 2009–2013 |
|---|---|---|---|
| Number of patients | 896 | 1401 | 959 |
| Number of centers | 102 | 99 | 99 |
| Age at transplant, years | |||
| median age (range) | 59 (22–80) | 59 (23–80) | 59 (28–78) |
| Gender | |||
| Male | 536 (60) | 841 (60) | 558 (58) |
| Region | |||
| US | 761 (85) | 1319 (94) | 950 (99) |
| Canada | 135 (15) | 82 (6) | 9 (<1) |
| Karnofsky Score | |||
| ≥ 90% | 533 (59) | 736 (53) | 519 (54) |
| < 90% | 316 (35) | 501 (36) | 390 (41) |
| Unknown | 47 (5) | 164 (12) | 50 (5) |
| Disease-related variables | |||
| Immunochemical subtype | |||
| IgG | 517 (58) | 762 (54) | 551 (57) |
| IgA | 190 (21) | 313 (22) | 196 (20) |
| Light chain | 147 (16) | 267 (19) | 183 (19) |
| Others | 9 (1) | 17 (1) | 15 (2) |
| Non-secretory | 28 (3) | 40 (3) | 14 (1) |
| Unknown Type | 5 (<1) | 2 (<1) | 0 |
| Serum creatinine at diagnosis | |||
| < 2 mg/dl | 595 (66) | 930 (66) | 664 (69) |
| ≥ 2 mg/dl | 135 (15) | 235 (17) | 147 (15) |
| Unknown | 166 (19) | 236 (17) | 148 (15) |
| Serum albumin at diagnosis | |||
| < 3.5 g/dl | 274 (31) | 416 (30) | 313 (33) |
| ≥ 3.5 g/dl | 388 (43) | 676 (48) | 486 (51) |
| Unknown | 234 (26) | 309 (22) | 160 (17) |
| ISS/DSS Stage III | |||
| Yes | 389 (43) | 538 (38) | 292 (30) |
| No | 487 (54) | 803 (57) | 588 (61) |
| Missing | 20 (2) | 60 (4) | 79 (8) |
| Transplant-related variables | |||
| Lines of chemotherapy | |||
| 1 | 606 (68) | 956 (68) | 779 (81) |
| 2 | 236 (26) | 358 (26) | 140 (15) |
| 3+ | 54 (6) | 87 (6) | 40 (4) |
| Chemotherapy | |||
| VTD | 9 (1) | 189 (13) | 62 (6) |
| RVD | 0 | 79 (6) | 467 (49) |
| CVD | 3 (<1) | 117 (8) | 134 (14) |
| VD | 3 (<1) | 86 (6) | 145 (15) |
| RD | 2 (<1) | 216 (15) | 124 (13) |
| TD | 184 (21) | 485 (35) | 15 (2) |
| VAD/similar | 695 (78) | 229 (16) | 12 (1) |
| Melphalan dose (mg/m2) for condition regimen | |||
| 140 | 167 (19) | 230 (16) | 100 (10) |
| 200 | 729 (81) | 1171 (84) | 859 (90) |
| Total No. of CD34 cells infused (×106/kg) | |||
| Median (range) | 6 (1–20) | 5 (1–20) | 4 (2–19) |
| Disease status at transplant | |||
| CR | 139 (16) | 177 (13) | 168 (18) |
| VGPR† | (NA) | (NA) | 330 (34) |
| PR | 635 (71) | 1069 (76) | 408 (43) |
| MR/NR/SD | 98 (11) | 113 (8) | 36 (4) |
| Relapse/Progression | 21 (2) | 42 (3) | 17 (2) |
| Unknown | 3 (<1) | 0 | 0 |
| Sensitivity to chemotherapy | |||
| Sensitive | 774 (86) | 1246 (89) | 906 (94) |
| Resistant | 119 (13) | 155 (11) | 53 (6) |
| Unknown | 3 (<1) | 0 | 0 |
| Time from diagnosis to transplant | |||
| < 6 months | 284 (32) | 391 (28) | 399 (42) |
| 6 – 12 months | 612 (68) | 1010 (72) | 560 (58) |
| Median follow-up of survivors (range), months | 120 (3–170) | 86 (3–129) | 39 (3–82) |
Legend: ISS, International Staging System; DSS, Durie Salmon Stage, VTD, boretezomib, thalidomide and dexamethasone; RVD, lenalidomide, bortezomib and dexamethasone; CVD, cyclophosphamide, bortezomib and dexamethasone; VD, bortezomib, dexamethasone; RD, lenalidomide, dexamethasone; TD, thalidomide, dexamethasone; VAD, vincristine, doxorubicin, and dexamethasone; CR, complete response; VGPR, very good partial response; PR, partial response; MR, minor response; NR, no response; SD, stable disease.
This was included only after 2008. Prior to 2008, VGPR patients would be included in PR group
Time of relapse and post-transplant outcomes
At the time of analysis, 17%, 20% and 46% of patients were alive without disease progression in the 2001–2004, 2005–2008, and 2009–2013 groups, respectively (Table 2). A higher proportion of patients in the most recent period had intent for planned post-AHCT consolidation and/or maintenance (72%) compared with 23% for the middle group and 6% for the earliest group. Median follow-up of survivors was 120 (3–170) months for 2001–2004, 86 (3–129) months for 2005–2008 and 39 (3–82) months for 2009–2013 groups. The median PFS from AHCT for the 2001–2004, 2005–2008, and 2009 to 2013 groups was 31.2, 29.9 and 30.4 months, respectively (Figure 1A). The median OS for the three groups were 65.5, 79.5 and 88.8 months (p<0.001), respectively, suggesting improving survival over the time period. (Figure 1B) A similar proportion of patients had relapsed within 12 and 24 months of AHCT during the three time periods (Table 2). Overall, 38%, 38% and 35% of patients had relapsed within 24 months of AHCT in the three groups respectively (Figure 1C).
Table 2.
Post-transplant characteristics
| Variable | 2001–2004 | 2005–2008 | 2009–2013 |
|---|---|---|---|
| Post-relapse salvage transplant | |||
| No salvage transplant | 702 (78) | 1097 (78) | 890 (93) |
| Salvage Auto transplant | 165 (18) | 254 (18) | 58 (6) |
| Salvage Allo transplant | 29 (3) | 50 (4) | 11 (1) |
| Time from transplant to relapse | |||
| NRM | 74 (8) | 83 (6) | 23 (2) |
| < 12 months | 155 (17) | 280 (20) | 444 (46) |
| 12 – 24 months | 201 (22) | 265 (19) | 198 (21) |
| 24 – 36 months | 141 (16) | 270 (19) | 138 (14) |
| 36 – 48 months | 94 (10) | 218 (16) | 80 (8) |
| >= 48 months | 62 (7) | 133 (9) | 41 (4) |
| No relapse and alive | 169 (19) | 152 (11) | 35 (4) |
| Planned post-HCT therapy | |||
| Novel agents (Lena+Bort/Lena/Bort)‡ | 30 (3) | 267 (19) | 695 (72) |
| Other agents | 29 (3) | 54 (4) | 6 (<1) |
| None | 818 (91) | 1010 (72) | 236 (25) |
| Missing | 19 (2) | 70 (5) | 22 (2) |
Legend NRM: non-relapse mortality
Figure 1.
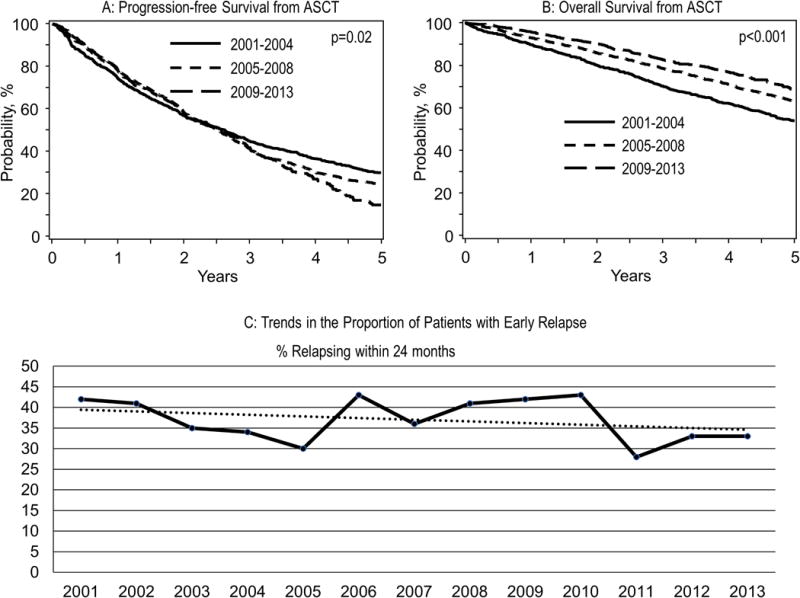
Panel A: Progression free survival from AHCT for the three groups of patients by the date of AHCT (2001-204, 2005–2008, 2009–2013).
Panel B: Overall survival from AHCT for the three groups of patients by the date of AHCT (2001-204, 2005–2008, 2009–2013)
Panel C: Trends in the proportion of patients with early relapse
We initially examined the impact of early post-AHCT relapse on OS of patients and how it has changed in the recent years. The OS from diagnosis was 44.7 months (95% CI: 42.5–48.2) for those relapsing within 24 months of AHCT compared with 113.7 months (95% CI:108.2–121.7) for those who had not relapsed within 24 months, reflecting the poor disease biology associated with early relapse (Figure 2A). Among those relapsing within 24 months, the median OS from diagnosis was 38.1, 48.1 and 48.3 for 2001–2004, 2005–2008, and 2009–2013 groups, respectively. For the remaining patients, the median OS from diagnosis was 102, 115.5 and 97.8 for the three time periods, respectively (p-value NS).
Figure 2.
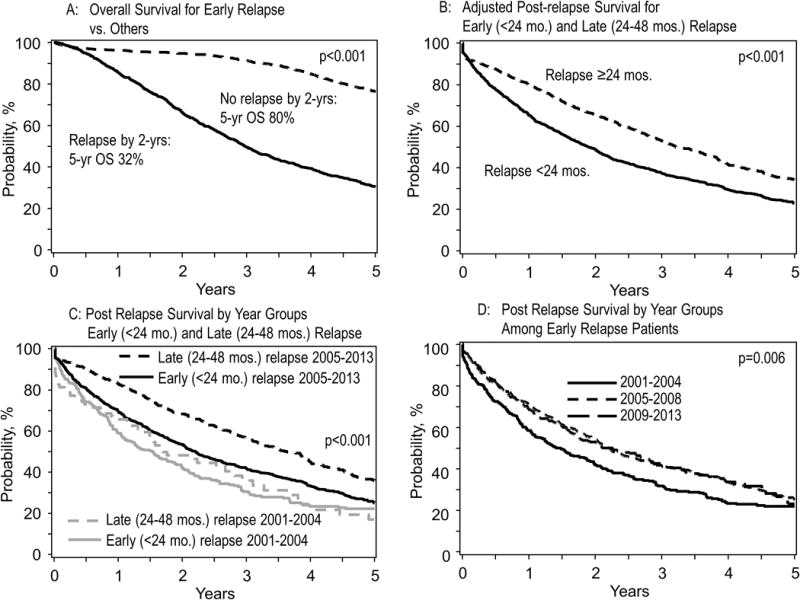
Panel A: Overall survival from diagnosis among patients with early relapse (< 24 months) and late relapse (>24 months)
Panel B: Post-relapse survival for early relapse patients (relapse within 24 months) compared to those with a late relapse
Panel C: Post-relapse survival for early relapse patients who relapsed within 24 months grouped by relapse year 2005
Panel D: Post-relapse survival for early relapse patients who relapsed within 24 months, grouped by the date of AHCT (2001-204, 2005–2008, 2009–2013)
Post-relapse outcomes
We evaluated the survival outcomes from relapse, comparing the outcomes of those relapsing within 24 months of AHCT and those relapsing beyond 24 months from AHCT or have not relapsed at last follow up. The baseline characteristics and the transplant related characteristics of these three groups of patients are as shown in Table 3. On multivariate, factors influencing early relapse included advanced MM stage [Hazard Ratio, (HR) 1.2; 95%CI: 1.0–1.3; p=0.02], chemo sensitivity (HR 0.8; 95%CI: 0.7–0.9, p=0.007), transplant after 2008 (HR 0.8; 95%CI: 0.7–0.98; p=0.02), and post-AHCT maintenance with novel agent (HR 0.8; 95%CI: 0.7–1.0, p=0.02) (Table 4).
Table 3.
Characteristics of patients grouped by timing of relapse*
| Variable | Relapse <24 months | Relapse after 24 months | No Relapse |
|---|---|---|---|
| Number of patients | 1156 | 984 | 893 |
| Age at transplant, years | |||
| median age (range) | 58 (31–80) | 60 (28–78) | 59 (22–80) |
| <50 | 216 (19) | 171 (18) | 167 (19) |
| 50–69 | 857 (75) | 747 (76) | 676 (76) |
| 70+ | 83 (7) | 66 (7) | 50 (6) |
| Gender | |||
| Male | 697 (60) | 596 (61) | 512 (57) |
| Karnofsky Performance Score | |||
| ≥ 90% | 623 (54) | 535 (54) | 506 (57) |
| < 90% | 436 (38) | 369 (38) | 331 (37) |
| Unknown | 97 (8) | 80 (8) | 56 (6) |
| Disease-related variables | |||
| Immunochemical subtype | |||
| IgG | 619 (54) | 565 (57) | 519 (58) |
| IgA | 300 (26) | 209 (21) | 152 (17) |
| Light chain | 186 (16) | 169 (17) | 191 (21) |
| Others | 17 (1) | 12 (1) | 9 (1) |
| Non-secretory | 31 (3) | 27 (3) | 20 (2) |
| Serum Creatinine at diagnosis | |||
| < 2 mg/dl | 787 (68) | 666 (68) | 597 (67) |
| ≥ 2 mg/dl | 196 (17) | 152 (15) | 131 (15) |
| Unknown | 173 (15) | 166 (17) | 165 (18) |
| Serum Albumin at diagnosis | |||
| < 3.5 g/dl | 384 (33) | 306 (31) | 254 (28) |
| ≥ 3.5 g/dl | 515 (45) | 473 (48) | 448 (50) |
| Unknown | 257 (22) | 205 (21) | 191 (21) |
| ISS/DS Stage III | |||
| Yes | 474 (41) | 367 (37) | 300 (34) |
| No | 629 (54) | 580 (59) | 554 (62) |
| Missing | 53 (5) | 37 (4) | 39 (4) |
| Transplant-related variables | |||
| Lines of chemotherapy | |||
| 1 | 783 (68) | 701 (71) | 683 (76) |
| 2 | 302 (26) | 223 (23) | 167 (19) |
| 3+ | 71 (6) | 60 (6) | 43 (5) |
| Chemotherapy | |||
| VTD | 98 (8) | 93 (9) | 64 (7) |
| RVD | 181 (16) | 94 (10) | 211 (24) |
| CVD | 80 (7) | 63 (6) | 72 (8) |
| VD | 76 (7) | 55 (6) | 79 (9) |
| RD | 134 (12) | 88 (9) | 103 (12) |
| TD | 234 (20) | 276 (28) | 152 (17) |
| VAD/similar | 353 (31) | 315 (32) | 212 (24) |
| Melphalan dose (mg/m2) for condition regimen | |||
| 140 | 198 (17) | 146 (15) | 128 (14) |
| 200 | 958 (83) | 838 (85) | 765 (86) |
| Total No. of CD34 cells infused (×106/kg) | |||
| Median (range) | 4.81 (1.00–19.11) | 5.34 (1.18–19.70) | 5.08 (1.19–19.56) |
| Disease status at transplant | |||
| CR | 142 (12) | 140 (14) | 171 (19) |
| PR | 860 (74) | 760 (77) | 652 (73) |
| MR/NR/SD | 108 (9) | 67 (7) | 56 (6) |
| Relapse/Progression | 45 (4) | 16 (2) | 14 (2) |
| Unknown | 1 (<1) | 1 (<1) | 0 |
| Sensitivity to chemotherapy | |||
| Sensitive | 1002 (87) | 900 (91) | 823 (92) |
| Resistant | 153 (13) | 83 (8) | 70 (8) |
| Unknown | 1 (<1) | 1 (<1) | 0 |
| Time from diagnosis to transplant | |||
| < 6 months | 368 (32) | 325 (33) | 301 (34) |
| 6 – 12 months | 788 (68) | 659 (67) | 592 (66) |
| Year of transplant | |||
| 2001–2004 | 331 (29) | 325 (33) | 198 (22) |
| 2005–2008 | 520 (45) | 503 (51) | 323 (36) |
| 2009–2013 | 305 (26) | 156 (16) | 372 (42) |
| Median follow-up of survivors (range), months | 75 (24–169) | 97 (25–170) | 60 (24–170) |
| Post-transplant characteristics | |||
| Post-relapse salvage transplant | |||
| No salvage transplant | 938 (81) | 699 (71) | 893 |
| Salvage AutoHCT | 159 (14) | 267 (27) | 0 |
| Salvage AlloHCT | 59 (5) | 18 (2) | 0 |
| Time from transplant to relapse | |||
| < 12 months | 624 (54) | 0 | |
| 12 – 24 months | 532 (46) | 0 | |
| 24 – 36 months | 0 | 392 (40) | |
| > 36 months | 0 | 236 (24) | |
| Planned post-HCT therapy | |||
| Novel agents (Lena+Bort/Lena/Bort) | 272 (24) | 254 (26) | 370 (41) |
| Other agents | 20 (2) | 39 (4) | 28 (3) |
| None | 813 (70) | 644 (65) | 485 (54) |
| Missing | 51 (4) | 47 (5) | 10 (1) |
Limited to patients with at least 24 months follow up if still alive
Legend VTD, boretezomib, thalidomide and dexamethasone; RVD, lenalidomide, bortezomib and dexamethasone; CVD, cyclophosphamide, bortezomib and dexamethasone; VD, bortezomib, dexamethasone; RD, lenalidomide, dexamethasone; TD, thalidomide, dexamethasone; VAD, vincristine, doxorubicin, and dexamethasone; CR, complete response; VGPR, very good partial response; PR, partial response; MR, minor response; NR, no response; SD, stable disease; HCT hematopoietic cell transplantation
Table 4.
Risk factors of early relapse (within 24 months)*
| Variable | Hazard Ratio | 95% Hazard Ratio Confidence Limits | P-value | ||
|---|---|---|---|---|---|
| Sensitivity to chemotherapy | Overall | 0.0071 | |||
| Resistant | 1.000 | ||||
| Sensitive | 0.824 | 0.715 | 0.949 | 0.0071 | |
| Year of transplant | Overall | 0.0179 | |||
| 2001–2008 | 1.000 | ||||
| 2009–2013 | 0.839 | 0.720 | 0.978 | 0.0179 | |
| ISS/DS Stage III At Diagnosis | Overall | 0.0132 | |||
| No | 1.000 | ||||
| Yes | 1.152 | 1.047 | 1.268 | 0.0037 | |
| Post-transplant maintenance | Overall | 0.0247 | |||
| No/Other agent | 1.000 | ||||
| Novel agent | 0.813 | 0.692 | 0.957 | 0.0180 | |
| Year of transplant # Post transplant maintenance | Overall | 0.0003 | |||
| 2001–2008, No/Other vs. Novel Agents | 1.219 | 1.035 | 1.436 | <.0001 | |
| 2009–2013, No/Other vs. Novel Agents | 1.256 | 1.025 | 1.538 | <.0001 | |
All patients were included in the analysis, including patients who didn’t relapse
Risk factors are the same for either early or late relapse
The median OS from the time of relapse was significantly inferior for the early relapse group compared with the late relapse groups; P<0.001 (Figure 2B, Table 5a). Next, we observed that while post-relapse survival of both early and late relapse were improved in the 2005–2013 period compared to 2001–2004, but improvements seemed greater for the late relapse group than for the early relapse group by year of transplant (Figure 2C). We then specifically examined the survival trends among the early relapse patients. Compared to patients transplanted in 2001–2004, patients with early relapse in the two later groups had improved OS from relapse (Figure 2D). The survival estimates over time for this group of patients are shown in Table 5b. We also examined the OS from relapse based on disease relapse before or after 2005. The median OS from relapse for those relapsing before 2005 was 16.4 months compared with 24.7 months for those relapsing after 2005; P <0.001. The survival estimates over time for this group of patients are shown in Table 5c.
Table 5.
| a. Post-relapse survival based on timing of relapse (early vs. late) | |||||
|---|---|---|---|---|---|
| Relapse within 24 months (N = 1156) | Relapse after 24 months (N=984) | ||||
| Outcomes | Number | Probability (95% CI) | Number | Probability (95% CI) | p-value |
| Post relapse survival | 1155 | 984 | <0.001 | ||
| 1-year | 65 (63–68)% | 80 (77–82)% | |||
| 2-year | 50 (47–53)% | 66 (62–69)% | |||
| 3-year | 38 (35–41)% | 53 (49–56)% | |||
| 4-year | 30 (27–33)% | 41 (38–45)% | |||
| b. Post-relapse survival trend of early relapse within 24 months during transplant year from 2001–2013 | |||||||
|---|---|---|---|---|---|---|---|
| 2001–2004 (N = 342) |
2005–2008 (N = 535) |
2009–2013 (N = 336) |
|||||
|
| |||||||
| Outcomes | Number | Probability (95% CI) | Number | Probability (95% CI) | Number | Probability (95% CI) | p-value |
| Overall Survival | 342 | 535 | 336 | 0.006 | |||
| 1-year | 58 (53–64)% | 70 (66–74)% | 68 (63–73)% | ||||
| 2-year | 42 (37–47)% | 54 (50–59)% | 53 (47–59)% | ||||
| 3-year | 32 (27–37)% | 42 (37–46)% | 41 (35–47)% | ||||
| 5-year | 22 (17–27)% | 25 (21–29)% | 23 (16–31)% | ||||
| c. Post-relapse survival of early relapse within 24 months by relapse year before and after 2005 | |||||
|---|---|---|---|---|---|
| Relapsed before 2005 (N = 284) |
Relapsed in or after 2005 (N = 929) |
||||
|
| |||||
| Outcomes | Number | Probability (95% CI) | Number | Probability (95% CI) | p-value |
| Post relapse Survival | 284 | 929 | 0.005 | ||
| 1-year | 59 (53–64)% | 69 (66–72)% | |||
| 2-year | 42 (36–48)% | 53 (50–57)% | |||
| 3-year | 30 (25–36)% | 41 (38–45)% | |||
| 4-year | 23 (18–29)% | 33 (29–36)% | |||
| 5-year | 22 (17–27)% | 25 (21–28)% | |||
We subsequently performed multivariate analysis to examine factors predicting for post-relapse OS among patients relapsing early or late after AHCT. Risk factors for post-relapse OS on multivariate analysis included early relapse (HR 1.4; 95%CI: 1.3–1.6, p<0.0001), Karnofsky <90 (HR 1.2; 95%CI: 1.1–1.4; p=0.007), stage III myeloma (HR 1.3; 95%CI: 1.1–1.4, p<0.0001), 2+ lines of chemotherapy (HR 1.2; 95%CI: 1.1–1.4, p=0.005), novel agent maintenance post-AHCT (HR 0.7; 95%CI: 0.6–0.9, p<0.0001), and IgA myeloma (HR 1.3; 95%CI: 1.1–1.5; p=0.0006) (Table 6).
Table 6.
Multivariate analysis of post-relapse survival
| Variable | Number | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|---|
|
| ||||
| Relapse Group | <0.0001 | |||
| -Late relapse | 628 | 1 | ||
| -Early relapse | 1213 | 1.43 | 1.26, 1.62 | |
|
| ||||
| Age at AHCT | 0.06 | |||
| -18–49 | 334 | 1 | ||
| -50–59 | 648 | 1.15 | 0.97, 1.37 | 0.10 |
| -60–69 | 727 | 1.23 | 1.04, 1.46 | 0.02 |
| -70+ | 132 | 1.33 | 1.03, 1.71 | 0.03 |
|
| ||||
| Stage III at Diagnosis | <0.0001 | |||
| -No | 1010 | 1 | ||
| -Yes | 747 | 1.28 | 1.13, 1.45 | <0.0001 |
| -Missing | 84 | 1.01 | 0.73, 1.40 | 0.89 |
|
| ||||
| Immunochemical subtype | 0.0006 | |||
| -IgG | 1010 | 1 | ||
| -IgA | 441 | 1.30 | 1.13, 1.49 | 0.0002 |
| -Light chain | 309 | 0.90 | 0.76, 1.06 | 0.20 |
| -Non-secretory/others | 73 | 1.00 | 0.74, 1.35 | 1.00 |
| -Missing | 4 | 0.83 | 0.27, 2.61 | 0.75 |
|
| ||||
| Karnofsky Performance Score at AHCT | 0.007 | |||
| -≥90% | 995 | 1 | ||
| -<90% | 686 | 1.21 | 1.07, 1.36 | 0.003 |
| -Missing | 160 | 1.07 | 0.86, 1.34 | 0.82 |
|
| ||||
| Lines of Pre-AHCT chemotherapy | 0.005 | |||
| -1 | 1273 | 1 | ||
| -2+ | 568 | 1.20 | 1.10, 1.35 | |
|
| ||||
| Post-transplant maintenance | <0.0001 | |||
| -No/Other agents | 1286 | 1 | ||
| -Novel agents | 473 | 0.73 | 0.63, 0.85 | <0.0001 |
| -Missing | 82 | 0.60 | 0.45, 0.80 | 0.0006 |
Legend AHCT, autologous hematopoietic cell transplantation
DISCUSSION
As the outcomes for MM patients continue to improve, disease heterogeneity has become increasingly evident, with nearly a quarter of patients continuing to have median overall survival of 2–3 years.15–20 These ‘high-risk’ patients are typically characterized by the presence of one or more cytogenetic abnormalities, but these abnormalities do not always account for the poor outcomes seen in some patients. Over the years, it has become apparent that patients with a short duration of response, particularly those relapsing early after AHCT, have a poor outcome, defining a functional high risk group of patients.9, 10, 12 Even in the current era with major improvements in the treatment approaches, especially more uniform application of highly effective regimens incorporating proteasome inhibitors and immunomodulatory drugs, AHCT continues to play a major role in the treatment of myeloma.2, 6, 7, 21 It is considered a standard component of the initial treatment approach for patients who can undergo this procedure. Much has changed in the context of transplant with better induction therapy, and uniform incorporation of post-transplant approaches such as consolidation and maintenance.4, 22–29 This study was undertaken to examine the clinical factors predicting early relapse in the face of these improvements in initial therapy and if the post relapse outcomes have improved with the increasing availability of novel classes of agents.
Examination of the baseline characteristics of the patients included in this study gives valuable information regarding the changing landscape of transplant utilization in North America, in the context of which the current results should be interpreted.2 The demographic characteristics of the patients going to transplant within 12 months of diagnosis has remained consistent over the study period. It is interesting to note a trend towards decreasing proportion of patients with International Staging System (ISS) stage 3 in the recent years, and may reflect an overall shift towards earlier treatment intervention among patients, a fact to be considered when interpreting the results.8 The type of induction regimens utilized pre-AHCT shows a significant shift towards use of proteasome inhibitor/immunomodulatory drug combinations such as VRD, which was used in nearly half of the patients in the recent group. The increased use of this regimen is consistent with the current recommendations based on results from the phase 3 trial of this regimen.30, 31 The impact of this shift in induction regimen likely explains the increasing proportion of patients coming into transplant with just one line of initial therapy in the most recent cohort, and with chemo sensitive disease. The quicker response seen with the newer regimens also likely explains the higher proportion of patients receiving transplant within 6 months of diagnosis in the most recent group. Finally, as expected a significantly higher proportion of patients were reported to have planned post-AHCT therapy in the form of maintenance with lenalidomide or bortezomib.
One of the striking findings of the current study is the lack of a substantial decrease in the proportion of patients who are relapsing within 24 months after AHCT; 38%, 38% and 35% during the three consecutive periods. Given that patients are likely to be going into transplant with more chemo sensitive disease, likely a deeper response and higher proportion with planned post AHCT therapy, the lack of improvement in this aspect of disease is intriguing as well as concerning. One potential explanation is that patients with genetically high-risk disease are being preferentially being steered towards transplant, but the proportion of ISS stage 3 disease being less in the recent years makes this explanation less likely. The proportion of patients progressing within 24 months of the transplant in the latest group is consistent with the findings from the phase 3 trial.28, 29 In the CALGB 100104 trial nearly 50% and 25% of patients in the observation and maintenance lenalidomide arms, respectively had relapsed within 24 months consistent with the 36% overall rate of progression seen here.29 It is possible that more patients whose disease achieve less than a VGPR after AHCT in the earlier years may have gone on to tandem AHCT and thus would be excluded from the current study.32 It is also possible this represents underlying biology, that is not being significant impacted by the alterations in the short course of induction therapy regimen or the post AHCT maintenance, but rather reflect an innate resistance to high-dose therapy. This is further underscored by the fact that the induction regimens were similar among the patients with a relapse within 24 months and those relapsing after 24 months. If that is indeed the case, it is important to understand the drivers and possibly predict the suboptimal outcomes such that we can design clinical trials for this high-risk patient population. The analysis does shed some light into the predictors of early relapse, information that could be utilized in designing clinical trials for this patient group.
Consistent with prior data, patients with early relapse continues to represent a poor prognosis subgroup of patients, who clearly need a different approach to their management.9, 12 In the current study, those relapsing within 24 months of transplant had a significantly shorter OS from the time of relapse, compared to those relapsing 24–48 months from AHCT. However, it is encouraging to see the improvement in survival of patients from the time of relapse in this high-risk group of patients over the years. The improvement is evident starting somewhere in the 2004–2008 period, and seems to be maintained over the subsequent years (Figure 2D). This improvement in post-relapse survival likely reflects the introduction of the newer drugs and more consistent availability of these drugs and the use of drug combinations in the setting of relapsed disease. However, the lack of further improvement between the 2005–08 cohort and the most recent group highlights the need for continued development of novel strategies. It is likely the effect of more recent improvements seen with newer drugs such as carfilzomib (FDA-approved in mid-2012), pomalidomide (FDA-approved in early 2013), ixazomib and monoclonal antibodies (FDA-approved later 2015) is likely not reflected here as our dataset covers practice in 2000–2013. In addition to the timing of relapse, several other risk factors for poor outcome following post AHCT relapse have been identified in the multivariate analysis. These include previously described factors such as the older age and poorer performance status, ISS stage III at diagnosis, IgA myeloma and >1 line of therapy prior to AHCT as well as lack of maintenance therapy following AHCT. It is certainly of interest that the post-relapse survival is higher among those who relapse on maintenance, suggesting lack of development of a more resistant disease phenotype among maintained patients and the availability of new classes of drugs in the recent years. This finding is consistent with what was seen in a recent meta-analysis of trials using post AHCT maintenance.33
The major limitation of our study is the lack of cytogenetic data on patients. Because the CIBMTR only collected cytogenetic data after 2008, it is not possible to obtain this information. Further, even in the 2008–2013 cohort, there may be heterogeneous FISH methodology, variable plasma cell enrichment, and possibility of false negative results. Patients defined as stage 3 include DSS and/or ISS 3 given that the ISS was only developed in 2004 and our data includes patients from the pre-ISS era. Lastly, we are unable to characterize whether the reported relapses were biochemical, clinical or radiological. Nevertheless, this study allows us, using a large database capturing the majority of MM AHCT activity in the region, to study systematically early relapsers after AHCT and assess changes in outcomes over time.
In conclusion, early relapse after initial therapy, in the context of an upfront transplant in this study, continues to be a risk and biology defining feature in myeloma. A relatively high constant proportion of patients with early relapse highlights critical aspects of biology that are not being addressed by current prognostic factors at diagnosis or current therapies. Identification of risk factors and well-designed laboratory studies of the tumor and microenvironment in these patients will lead to further improvements over time. The improved outcomes from relapse is encouraging and this is likely to improve over time with introduction of newer therapies.
Supplementary Material
Acknowledgments
DISCLOSURES
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government
*Corporate Members
This publication is funded in part by the Research and Education Program Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and by KL2TR001438 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences (D’Souza, A). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICTS OF INTERESTS
The authors have no conflicts of interests to report.
References
- 1.Laubach J, Kumar S. Management of Transplant-Eligible Patients with Newly Diagnosed Multiple Myeloma. Cancer Treat Res. 2016;169:145–167. doi: 10.1007/978-3-319-40320-5_9. [DOI] [PubMed] [Google Scholar]
- 2.Costa LJ, Zhang M-J, Zhong X, Dispenzieri A, Lonial S, Krishnan A, et al. Trends in Utilization and Outcomes of Autologous Transplantation as Early Therapy for Multiple Myeloma. Biology of Blood and Marrow Transplantation. 2013;19(11):1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. The New England journal of medicine. 2017;376(14):1311–1320. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. The New England journal of medicine. 2014;371(10):895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 5.Hari PN, McCarthy PL. Multiple myeloma: future directions in autologous transplantation and novel agents. Biol Blood Marrow Transplant. 2013;19(1 Suppl):S20–25. doi: 10.1016/j.bbmt.2012.11.002. e-pub ahead of print 2013/01/11. [DOI] [PubMed] [Google Scholar]
- 6.Schiffer CA, Zonder JA. Transplantation for Myeloma – Now or Later? N Engl J Med. 2017;376(14):1378–1379. doi: 10.1056/NEJMe1700453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Souza A, Zhang MJ, Huang J, Fei M, Pasquini M, Hamadani M, et al. Trends in pre- and post-transplant therapies with first autologous hematopoietic cell transplantation among patients with multiple myeloma in the United States, 2004–2014. Leukemia. 2017 doi: 10.1038/leu.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimenez-Zepeda VH, Reece DE, Trudel S, Chen C, Tiedemann R, Kukreti V. Early relapse after single auto-SCT for multiple myeloma is a major predictor of survival in the era of novel agents. Bone Marrow Transplant. 2015;50(2):204–208. doi: 10.1038/bmt.2014.237. [DOI] [PubMed] [Google Scholar]
- 10.Majithia N, Rajkumar SV, Lacy MQ, Buadi FK, Dispenzieri A, Gertz MA, et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia. 2016;30(11):2208–2213. doi: 10.1038/leu.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palumbo A, Bringhen S, Falco P, Cavallo F, Ambrosini MT, Avonto I, et al. Time to first disease progression, but not beta2-microglobulin, predicts outcome in myeloma patients who receive thalidomide as salvage therapy. Cancer. 2007;110(4):824–829. doi: 10.1002/cncr.22855. e-pub ahead of print 2007/06/28. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. 2008;42(6):413–420. doi: 10.1038/bmt.2008.180. e-pub ahead of print 2008/07/01; doi: bmt2008180 [pii] 10.1038/bmt.2008.180. [DOI] [PubMed] [Google Scholar]
- 13.Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI, et al. Salvage second hematopoietic cell transplantation in myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(5):760–766. doi: 10.1016/j.bbmt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 15.Dispenzieri A. Myeloma: management of the newly diagnosed high-risk patient. Hematology Am Soc Hematol Educ Program. 2016;2016(1):485–494. doi: 10.1182/asheducation-2016.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper R, van Duin M, van Vliet MH, Broijl A, van der Holt B, El Jarari L, et al. Prediction of high- and low-risk multiple myeloma based on gene expression and the International Staging System. Blood. 2015;126(17):1996–2004. doi: 10.1182/blood-2015-05-644039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonial S, Boise LH, Kaufman J. How I treat high-risk myeloma. Blood. 2015;126(13):1536–1543. doi: 10.1182/blood-2015-06-653261. [DOI] [PubMed] [Google Scholar]
- 18.Scott EC, Hari P, Sharma M, Le-Rademacher J, Huang J, Vogl D, et al. Post-Transplant Outcomes in High-Risk Compared with Non-High-Risk Multiple Myeloma: A CIBMTR Analysis. Biol Blood Marrow Transplant. 2016;22(10):1893–1899. doi: 10.1016/j.bbmt.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voorhees PM, Usmani SZ. The role of high-dose melphalan and autologous stem cell transplant in the rapidly evolving era of modern multiple myeloma therapy. Clin Adv Hematol Oncol. 2016;14(9):719–728. [PubMed] [Google Scholar]
- 22.McCarthy PL, Einsele H, Attal M, Giralt S. The emerging role of consolidation and maintenance therapy for transplant-eligible multiple myeloma patients. Expert review of hematology. 2014;7(1):55–66. doi: 10.1586/17474086.2014.878645. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan A, Vij R, Keller J, Dhakal B, Hari P. Moving Beyond Autologous Transplantation in Multiple Myeloma: Consolidation, Maintenance, Allogeneic Transplant, and Immune Therapy. Am Soc Clin Oncol Educ Book. 2016;35:210–221. doi: 10.14694/EDBK_159016. [DOI] [PubMed] [Google Scholar]
- 24.Mohty M, Richardson PG, McCarthy PL, Attal M. Consolidation and maintenance therapy for multiple myeloma after autologous transplantation: where do we stand? Bone marrow transplantation. 2015;50(8):1024–1029. doi: 10.1038/bmt.2015.83. [DOI] [PubMed] [Google Scholar]
- 25.Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(25):2712–2717. doi: 10.1200/JCO.2013.54.8164. [DOI] [PubMed] [Google Scholar]
- 26.Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28(3):690–693. doi: 10.1038/leu.2013.335. [DOI] [PubMed] [Google Scholar]
- 27.Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(24):2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 28.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. e-pub ahead of print 2012/05/11. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–527. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, et al. Management of Newly Diagnosed Symptomatic Multiple Myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines 2013. Mayo Clin Proc. 2013;88(4):360–376. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349(26):2495–2502. doi: 10.1056/NEJMoa032290. e-pub ahead of print 2003/12/26; doi: 10.1056/NEJMoa032290 349/26/2495 [pii] [DOI] [PubMed] [Google Scholar]
- 33.McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017:JCO2017726679. doi: 10.1200/JCO.2017.72.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


