Abstract
Objectives
To determine the prevalence of sarcopenia in older men with peripheral arterial disease (PAD) and to compare to a subset of the group to age, race, sex, and body mass index (BMI)-matched non-PAD control counterparts. We also sought to compare the functional status of those with PAD with and without sarcopenia.
Design
Cohort study
Setting
A Veterans Affairs medical center
Participants
Sedentary, community dwelling men age 50+ years with a confirmed diagnosis of PAD (N=108; 44% black; BMI 27.8 ± 0.4 kg/m2; ABI:0.62 ± 0.01; mean ±SEM).
Interventions
Not applicable
Main Outcomes
Dual-energy x-ray absorptiometry scans were used to assess appendicular lean mass (ALM) and determine the prevalence of sarcopenia by ALM/height2 (ALM/ht2). Treadmill tests were used to determine claudication onset time (COT), peak walking time (PWT), and claudication recovery time (CRT). Six-minute walk distance (6MWD) was also measured.
Results
Sarcopenia prevalence in our PAD cohort was 25%. The PAD subset (N=42) matched with control counterparts for race, sex, age, and BMI had higher prevalence rates compared with their non-PAD counter parts (23.8% vs 2.4%, P<0.05). Individuals with sarcopenia (N=28) had lower 6MWD (p<0.05; 326± 18.8 vs 380 ± 9.7 meters) and higher CRT (p<0.05; 592 ± 98 vs 395 ± 29 seconds) compared to individuals with PAD but without sarcopenia (N=80). There was no difference in COT or PWT between the PAD groups.
Conclusions
Men with PAD demonstrate a high prevalence of sarcopenia. Those with sarcopenia and PAD demonstrate decreased mobility function.
Keywords: Muscle, Sarcopenia, Peripheral Arterial Disease, Rehabilitation
Sarcopenia, or the age related loss of muscle mass and strength, is reported to affect up to 13% of individuals age 60–70 years1,2 and increases in prevalence as age increases.3 Among older adults the presence of sarcopenia is associated with an increase in physical disability,4 poor quality of life,5 and an increase in all-cause mortality independent of age, sex, and comorbidity.3,6 Further, the presence of sarcopenia increases direct health care costs and is estimated to cost the United States 18.5 billion per year.7 Risk factors for sarcopenia include low levels of physical activity, poor nutrition, and high co-morbid disease burden including the presence of diabetes or cardiovascular disease.8
Peripheral arterial disease (PAD) is an age related condition affecting up to 20% of older adults.9,10 PAD results in decreased blood flow to the lower extremities and those with PAD experience exertional leg symptoms including leg pain and fatigue during activity.10,11 As a result of the PAD process and chronic ischemia to the calf muscles, individuals with PAD demonstrate decreased calf muscle area and strength12–15 and low mobility16 and low physical activity.17 Previous studies show that adults with PAD have an 8% decrease in calf muscle area compared to older adults without PAD.13 While the calf muscle is known to be the affected end organ of PAD, it is likely that the sedentary lifestyle and comorbid conditions that affect those with PAD are likely to have whole body effects such as systemic muscle mass loss. Due to the adverse effects and increased health risks in the presence of sarcopenia, it is clinically important to have a better understanding of the prevalence of sarcopenia in those with PAD. Therefore, the purpose of this study was to examine the prevalence of sarcopenia in a group of older men with PAD. Because both sarcopenia and PAD are also known to result in decreased mobility function, we also wanted to compare mobility function in older men with PAD with and without concomitant sarcopenia. We hypothesized that a high prevalence of sarcopenia would exist among older men with PAD and that men with combined PAD and sarcopenia would demonstrate lower mobility levels than those with PAD alone.
Methods
Participant Selection
Sedentary, community dwelling men, over the age of 50 years were recruited via local advertisements in the Baltimore/Washington DC area as well as from the Baltimore VA vascular surgery clinic. All participants experienced intermittent claudication during a screening treadmill test, and had a diagnosis of PAD, or a resting ankle-brachial index (ABI) <0.90,18 with evidence of a decline in ABI > 20% after exercise were recruited.18 Only those with body composition data were included in the analyses. Exclusion criteria included claudication pain at rest, exercise tolerance limited by factors other than claudication pain (e.g., severe coronary artery disease), substance abuse, dementia, or any poorly controlled medical conditions that would limit participation in an exercise test (e.g., pulmonary disease or severe arthritis).
To examine the prevalence of sarcopenia in those with PAD and control for potential confounders, a matched pair analysis was performed. Based on the report from Baumgartner et al,19 we estimated that n=42 per group were required to detect an effect size of 1 standard deviation in our primary outcomes of ALM and ALM/h2. Individuals with PAD were matched with sedentary control participants who did not have PAD on the basis of race, sex, age ± 5 years and BMI ± 3.0 kg/m2. If more than one patient with PAD was available for matching with a control based on age the participant with the closest BMI was selected as the match. Control participants had the same exclusion criteria as individuals with PAD and were participating in studies examining the metabolic responses to exercise training and weight loss. Control participants were also recruited from with the use of local advertisements and from the Baltimore VA clinics. All data in healthy controls was obtained prior to any intervention. Healthy controls were sedentary (less than 20 minutes of exercise 2-times/week for at least the last 6 months) nonsmokers and had no history of stroke, heart failure, liver, kidney, or lung disease. Healthy individuals as well as those with PAD underwent a comprehensive medical exam including a history and physical by a licensed medical provider to ensure they met all inclusion/exclusion criteria prior to participation in the study. Written informed consent was obtained from each participant and all procedures were approved by the Institutional Review Board at the University of Maryland, Baltimore.
Body Composition and Determination of Sarcopenia
Height in meters and weight in kilograms were measured and used to determine body mass index (BMI; kg/m2). Whole body DXA scans were used to determine, total body, leg, arm, trunk, and appendicular lean mass, fat mass, and percent body fat.20,21 The presence of sarcopenia was determined by a ratio of appendicular lean mass (ALM) to height in meters squared (ALM/h2). Sarcopenia was considered to be present when ALM/ht2<7.26 kg/m2.19
Exercise and Functional Tests in participants with PAD
Maximal Treadmill Test
After a resting ankle brachial index (ABI) was obtained, participants with PAD performed a progressive, graded treadmill protocol (2 mph, 0% grade with 2% increase every 2 minutes) until maximal claudication pain was reached.22 Variables recorded from the treadmill test include: 1) Claudication onset time (COT), defined as the walking time that participants first experienced claudication pain, 2) peak walking time (PWT), defined as the walking time at which ambulation could not continue due to maximal claudication pain, and 3) claudication recovery time (CRT), the time after cessation of walking until no pain was felt.
Six-minute walk distance
Participants completed an overground 6-minute walk test on a day separate from the treadmill tests, as described previously, and the total distance achieved (6MWD) was recorded.23,24
Walking Impairment Questionnaire (WIQ)
Self-reported ambulatory ability was assessed with the WIQ. The WIQ is a questionnaire that asks participants to rate the degree of difficulty in walking specific distances (ranging from walking indoors to 1500 feet), speeds (walking to jogging), and climbing flights of stairs (ranging from one to three flights) on a zero (unable to do it) to four (able to do it without difficulty) scale.25 Each response is weighted based on the difficulty of the task and the score is determined by dividing the weighted answer by the maximum possible score and multiplying by 100. Scores range from 0–100 with lower scores indicating greater difficulty with walking.
Statistical Analyses
All statistical analyses were performed using IBM SPSS Statistics v 20.0 (IBM, Armonk, NY). Data were initially assessed to ensure normal distribution. All body composition data was normally distributed. Physical function tests were not normally distributed so a Mann-Whitney U test was used to compare physical function (COT, PWT, CRT, 6MWD, and WIQ) between individuals with PAD with and without sarcopenia. Chi-square tests were used to compare the prevalence of sarcopenia in black and white males as well as between those with PAD and matched controls. Data are presented as means ± SEM. Significance was set at P≤0.05.
Results
Subject Characteristics
One-hundred and forty-four men with PAD were initially identified; thirty-six were missing DXA data leaving 108 for analysis. As seen in Table 1, participants with PAD were diverse in terms of age (range 53–88 years), BMI (range 18.5–41.7 kg/m2), and PAD severity with an average resting ABI of 0.65 (range 0.28–0.9) indicating moderate PAD. A large portion of those with PAD were current smokers (41.7%) and approximately one-third (31.3%) had a diagnosis of diabetes. The group was racially mixed with 55.6% white (N=60) and 44.4% (N=48) black. Race effects on all variables were assessed. Total body (black: 51.6 ± 1.1 kg white: 55.2 ± 0.9; P=0.01) and trunk lean mass (black: 28.1 ± 0.6 kg; white: 31.0 ± 0.5 kg; P<0.001) were different between black and white individuals with PAD. There was a similar incidence of sarcopenia between black and white participants (white = 23.3%; black = 29.1%; P=0.49) and with a total prevalence of sarcopenia of 25.9% (N=28/108). Due to the potential relationship between sarcopenia and diabetes, all individuals with diabetes were removed from the analysis. This did not significantly change the results and we found the overall prevalence of sarcopenia was 31.9% (N=22/69) in those with PAD but without diabetes.
Table 1.
Comparison of Function in Men with PAD + Sarcopenia versus PAD
| Total Group (N=108) |
Total Group Range | PAD (N=80) |
PAD+ Sarcopenia (N=28) |
|
|---|---|---|---|---|
| Age (years) | 68.7 ± 0.6 | 53–88 | 68.7 ± 0.7 | 68.4 ± 1.2 |
| Weight (kg) | 83.0 ± 1.5 | 56.3–127.0 | 86.2 ± 1.6 | 73.9 ± 2.8* |
| BMI (kg/m2) | 27.8 ± 0.4 | 18.6–41.7 | 29.0 ± 0.4 | 24.3 ± 0.8* |
| ABI | 0.62 ± 0.01 | 0.28–0.90 | 0.63 ± 0.02 | 0.60 ± 0.03 |
| Body Fat (%) | 29.4 ± 0.7 | 6.9–43.9 | 30.2 ± 0.7 | 27.0 ± 1.5* |
| Total Body Lean Mass (kg) | 53.6 ± 0.7 | 34.0–72.0 | 55.8 ± 0.8 | 47.4 ± 1.0* |
| Arm Lean Mass (kg) | 6.5 ± 0.1 | 3.9–9.4 | 6.9 ± 0.1 | 5.6 ± 0.1* |
| Leg Lean Mass (kg) | 17.4 ± 0.3 | 10.8–25.1 | 18.2 ± 0.3 | 14.9 ± 0.4* |
| Trunk Lean Mass (kg) | 29.7 ± 0.4 | 19.2–42.8 | 30.7 ± 0.4 | 26.9 ± 0.6* |
| ALM (kg) | 23.9 ± 0.4 | 14.8–34.5 | 25.0 ± 0.4 | 20.5 ± 0.5* |
| ALM/ht2 (kg/m2) | 8.0 ± 0.1 | 5.0–11.1 | 8.4 ± 0.1 | 6.7 ± 0.1* |
| COT (sec) | 168 ± 12.2 | 28–805 | 185 ± 14.2 | 149 ± 23.7 |
| PWT (sec) | 419 ± 22.7 | 107–1201 | 436 ± 24.9 | 406 ± 53.3 |
| CRT (sec) | 441 ± 30.6 | 90–1621 | 395 ± 28.5 | 592 ± 97.9* |
| 6MWD (meters) | 367 ± 8.9 | 169–551 | 380 ± 9.7 | 326 ± 18.8* |
| WIQ Distance (%) | 32.3 ± 3.1 | 0–100 | 36.4 ± 3.5 | 24.5 ± 6.3 |
| WIQ Speed (%) | 37.4 ± 2.8 | 0–100 | 38.7 ± 3.1 | 34.4 ± 6.4 |
| WIQ Stairs (%) | 49.4 ± 3.1 | 0–100 | 49.5 ± 3.6 | 50.0 ± 6.5 |
| Sarcopenia (%) | 25.9% | – | – | – |
Significant (P≤0.05) difference between individuals diagnosed with peripheral artery disease (PAD) with and without sarcopenia; BMI: Body Mass Index; ABI: Ankle Brachial Index; ALM: Appendicular lean mass; ALM/ht2; Appendicular lean mass/height2; COT: Claudication Onset Time; PWT: Peak Walking Time; CRT: Claudication Recovery Time; 6MWD: six-minute walk distance; WIQ: Walking Impairment questionnaire.
Sarcopenic vs. Non-sarcopenic PAD patients
Sarcopenia PAD patients had significantly lower weight and BMI’s compared to those who had PAD without sarcopenia (Table 1, P<0.001). There was no difference in age between the groups. While almost all measures of function were lower in those with PAD and sarcopenia, only CRT and 6MWD reached statistical significance (table 1). Men with PAD and sarcopenia took approximately 37% longer to CRT after the cessation of the treadmill exercise test (P=0.05) and had approximately 14% lower 6MWD during the six-minute walk test (P=0.02) compared to those with PAD but without sarcopenia (Figure 1). Given the difference in body weight between PAD patients with and without sarcopenia, exploratory analyses were also run with weight as a co-variate; however, this adjustment did not significantly affect the results (data not shown).
Figure 1.
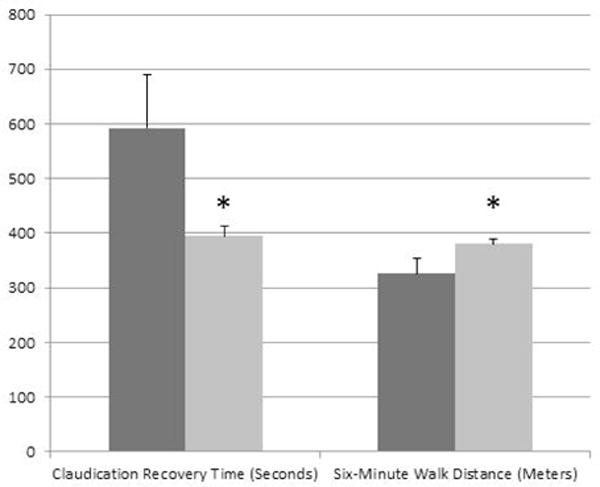
Differences in the claudication recovery time and six-minute walk distance (mean ± standard error) in men with PAD + Sarcopenia (dark bars) vs PAD (light bars). Significant differences were seen between the groups for claudication recovery time and six-minute walk distance. *P<0.05.
PAD vs. Controls
In the matched analysis, the non-PAD controls were well matched (N=42 per group) in terms of age and BMI (Table 2, P >0.6 for all). After matched pair analysis using ALM/ht2, a higher prevalence of sarcopenia was found in men with PAD than controls (Table 2; P=0.004). One out of 42 individuals in the matched controls (2.4%) and 10 out of 42 individuals (23.8%) with PAD met the definition for sarcopenia. Controls were found to have 9.9% higher leg lean mass (Table 2; p<0.001), and 8.5% higher total body lean mass (p=0.002) when compared to men with PAD. We further found a 6.7% difference in trunk lean mass between individuals with and without PAD (Table 2; p=0.005) indicating differences extended beyond that of a mere loss of leg lean mass in those with PAD.
Table 2.
Comparison of individuals with PAD and healthy controls without PAD
| PAD (N=42) |
Controls (N=42) |
|
|---|---|---|
| Age (years) | 65.4 ± 0.8 | 65.7 ± 0.9 |
| Weight (kg) | 89.7 ± 2.3 | 92.7 ± 2.0 |
| BMI (kg/m2) | 29.6 ± 0.7 | 29.9 ± 0.6 |
| Body Fat (%) | 30.8 ± 0.9 | 32.5 ± 2.3 |
| Total Body Lean Mass (kg) | 56.5 ± 1.0 | 61.1 ± 0.9* |
| Arm Lean Mass (kg) | 6.9 ± 0.2 | 7.3 ± 0.2 |
| Leg Lean Mass (kg) | 18.1 ± 0.4 | 20.0 ± 0.4* |
| Trunk Lean Mass (kg) | 31.5 ± 0.4 | 33.7 ± 0.3* |
| ALM (kg) | 25.0 ± 0.6 | 27.3 ± 0.5* |
| ALM/ht2 (kg/m2) | 8.2 ± 0.2 | 8.8 ± 0.2* |
| Sarcopenia (%) | 23.8 | 2.4* |
Significant (P≤0.05) difference between individuals diagnosed with peripheral artery disease (PAD) and matched controls; BMI: Body Mass Index; ABI: Ankle Brachial Index; ALM/ht2; Appendicular lean mass/height2.
Discussion
Our results demonstrate a high prevalence of sarcopenia of approximately 25% in males with PAD. This rate is especially high when considering the relativity low median age of 68 years in our group as a whole. While previous research documents decreased calf muscle volume in those with PAD,13 this is the first study we are aware of that examines the prevalence of sarcopenia in this population. Confirming our hypothesis, we found longer claudication recovery times and lower six-minute walk distance in individuals with PAD and sarcopenia compared to those with only PAD, indicating a possible increased risk for accelerated mobility loss.
The 25% prevalence of sarcopenia in this group of PAD patients is substantially higher than the 2–13% previously reported among various groups of community dwelling males,26–33 and is in fact higher than the rates reported in studies of older hospitalized or institutionalized males.6,34 For example, Smoliner et al,34 found a prevalence of sarcopenia of 15.2% among hospitalized men age 80+ years and Landi et al6 reported a prevalence of 15.6% among institutionalized males age 70+ years. While both of these studies included low mobility function in their definition, it is likely that our prevalence of sarcopenia would have either remained the same, or possibly even increased, with the addition of gait speed in our definition of sarcopenia as multiple studies have documented low gait-speed in those with PAD.35,36 Indeed when we compare the prevalence of sarcopenia in our group of relatively younger PAD patients to age, sex, and BMI matched controls, we found an almost ten times greater presence of sarcopenia in men with PAD.
We also found significant differences between men with PAD and matched controls in total body, leg, and trunk lean mass. This is an important finding as it indicates an accelerated loss of whole body muscle mass in individuals with PAD. While previous work examined calf muscle area and density in adults with PAD,13,15,37 this is the first study we are aware of that compared total body lean mass in matched men with and without PAD. Our finding of reduced trunk lean mass suggests that those with PAD and sarcopenia may be at an increased future risk for balance and mobility problems.38,39 This is supported by our finding that men with PAD and sarcopenia demonstrated significantly decreased function in PAD specific mobility measures including CRT and 6MWD. Sarcopenic individuals with PAD walked ~15% shorter distance during the six-minute walk compared to individuals with PAD but without sarcopenia, and ~43% less than age matched norms reported in the literature.40,41 This is in agreement with previous research that shows those with sarcopenia are at risks for mobility declines4 and indicates that PAD and sarcopenia may act synergistically as a threats to mobility function.
The mechanisms behind the high prevalence of sarcopenia in this population is unknown but may result from a combination of the chronic nature of the PAD process as well as the decreased physical activity typically reported in this population.17,42,43 Sedentary behavior is a known risk factor for sarcopenia and individuals with PAD demonstrate lower activity levels than individuals of a similar age.17,42,43 Another potential explanation is a decrease in capillary density. Low skeletal muscle capillarization is associated with sarcopenia in sedentary older adults,21 and is also found in PAD,44 thereby increasing the likelihood that low capillary density is associated with sarcopenia in PAD. Individuals with PAD are also frequently diagnosed with multiple co-morbidities that are risk factors for sarcopenia, including some we observed in our sample population, such as diabetes and a history of smoking.
Study Limitations
This study had several limitations. There is currently no universally accepted definition of sarcopenia and while we measured muscle mass by DXA, we were unable to account for gait-speed or grip strength criteria utilized in definitions of sarcopenia by the European Working Group on Sarcopenia in Older People.45 However, given that our 6MWD was ~40% lower than age matched norms,40,41 and previous research documents low-gait speed in individuals with PAD,46 it is likely that our population has a slow gait-speed. Our study also included only men from one VA hospital and as such our findings cannot be extended to females with PAD and may not represent male Veterans as a whole. Prevalence of sarcopenia may be related to sex as prevalence is found to differ by age and is generally reported to be higher in women < 75 years,47 thus sex should be considered in future studies examining the prevalence of sarcopenia among older adults with PAD. Our controls were selected from studies that purposefully excluded many co-morbid conditions. Thus, we also did not account for multiple co-morbid conditions found in men with PAD that may be potentially driving their higher prevalence of sarcopenia. However, though when we removed those individuals with diabetes from the analysis, the prevalence of sarcopenia remained the same or even slightly higher among those with PAD. Finally we acknowledge that while we included over 100 men with PAD we may have been underpowered to detect differences in COT, PWT, and the WIQ between the PAD and PAD + Sarcopenia groups.
Conclusions
In conclusion, there is a 10-fold higher prevalence of sarcopenia in men with PAD than in matched controls, and those with PAD and sarcopenia demonstrate lower mobility function than PAD men without sarcopenia, suggesting that the combination of PAD and sarcopenia may compound mobility dysfunction. Future studies are needed to examine interventions that can be directed at the prevention and treatment of sarcopenia in PAD as well as the mechanisms by which sarcopenia develops in this population.
Acknowledgments
This work was presented at the World Congress of Gerontology and Geriatrics 2017, San Francisco, California. This research was supported by the University of Maryland Claude D. Pepper Center (P30-AG-12583), the Baltimore Veterans Affairs Medical Center Geriatric Research, Education and Clinical Center (GRECC), and the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Award number I21RX001927. OA was supported by a VA Career Development Award (IK2RX001788), A.S.R. was supported by a VA Senior Research Career Scientist Award, and S.J.P was supported by a Paul B. Beeson Patient-Oriented Research Career Development Award in Aging (NIH K23-AG040775 and AFAR), MSC was supported by a VA Career Development Award (IK2RX000944).
Abbreviations
- ABI
Ankle brachial index
- ALM
Appendicular lean mass
- ALM/ht2
Appendicular lean mass/height2
- BMI
Body mass index
- COT
Claudication onset time
- CRT
Claudication recovery time
- DXA
Dual-energy x-ray absorptiometry
- PAD
Peripheral arterial disease
- PWT
Peak walking time
- WIQ
Walking impairment questionnaire
- 6MWD
Six-minute walk distance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest
References
- 1.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452–6. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 3.Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67:48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 4.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo T, Yu S, Visvanathan R. Systematic Literature Review on the Relationship Between Biomarkers of Sarcopenia and Quality of Life in Older People. J Frailty Aging. 2016;5:88–99. doi: 10.14283/jfa.2016.93. [DOI] [PubMed] [Google Scholar]
- 6.Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13:121–6. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7. doi: 10.1097/MCO.0b013e328333c1c1. [DOI] [PubMed] [Google Scholar]
- 9.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163:1469–74. doi: 10.1001/archinte.163.12.1469. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Criqui MH, Greenland P, et al. Leg strength in peripheral arterial disease: associations with disease severity and lower-extremity performance. J Vasc Surg. 2004;39:523–30. doi: 10.1016/j.jvs.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Ferrucci L, Guralnik J, et al. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–55. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott MM, Guralnik JM, Ferrucci L, et al. Physical activity, walking exercise, and calf skeletal muscle characteristics in patients with peripheral arterial disease. J Vasc Surg. 2007;46:87–93. doi: 10.1016/j.jvs.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott MM, Liu K, Tian L, et al. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. J Am Coll Cardiol. 2012;59:1159–67. doi: 10.1016/j.jacc.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep. 2013;15:347. doi: 10.1007/s11886-013-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–83. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 18.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AS, Ivey FM, Serra MC, Hartstein J, Hafer-Macko CE. Sarcopenia and Physical Function in Middle-Aged and Older Stroke Survivors. Arch Phys Med Rehabil. 2016 doi: 10.1016/j.apmr.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prior SJ, Ryan AS, Blumenthal JB, Watson JM, Katzel LI, Goldberg AP. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71:1096–101. doi: 10.1093/gerona/glw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner AW, Katzel LI, Sorkin JD, et al. Improved functional outcomes following exercise rehabilitation in patients with intermittent claudication. J Gerontol A Biol Sci Med Sci. 2000;55:M570–7. doi: 10.1093/gerona/55.10.m570. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–11. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 24.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–74. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regensteiner J, Steiner J, Panzer R. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Bio. 1990;2:142–52. [Google Scholar]
- 26.Lera L, Albala C, Sanchez H, et al. Prevalence of Sarcopenia in Community-Dwelling Chilean Elders According to an Adapted Version of the European Working Group on Sarcopenia in Older People (EWGSOP) Criteria. J Frailty Aging. 2017;6:12–7. doi: 10.14283/jfa.2016.117. [DOI] [PubMed] [Google Scholar]
- 27.Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK, Group IR Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc. 2013;14:528 e1–7. doi: 10.1016/j.jamda.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Legrand D, Vaes B, Mathei C, Swine C, Degryse JM. The prevalence of sarcopenia in very old individuals according to the European consensus definition: insights from the BELFRAIL study. Age Ageing. 2013;42:727–34. doi: 10.1093/ageing/aft128. [DOI] [PubMed] [Google Scholar]
- 29.Lin CC, Lin WY, Meng NH, et al. Sarcopenia prevalence and associated factors in an elderly Taiwanese metropolitan population. J Am Geriatr Soc. 2013;61:459–62. doi: 10.1111/jgs.12129. [DOI] [PubMed] [Google Scholar]
- 30.Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013;42:378–84. doi: 10.1093/ageing/afs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott D, Hayes A, Sanders KM, Aitken D, Ebeling PR, Jones G. Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos Int. 2014;25:187–93. doi: 10.1007/s00198-013-2431-5. [DOI] [PubMed] [Google Scholar]
- 32.Volpato S, Bianchi L, Cherubini A, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. 2014;69:438–46. doi: 10.1093/gerona/glt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida D, Suzuki T, Shimada H, et al. Using two different algorithms to determine the prevalence of sarcopenia. Geriatr Gerontol Int. 2014;14(Suppl 1):46–51. doi: 10.1111/ggi.12210. [DOI] [PubMed] [Google Scholar]
- 34.Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc. 2014;15:267–72. doi: 10.1016/j.jamda.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Gardner AW, Forrester L, Smith GV. Altered gait profile in subjects with peripheral arterial disease. Vasc Med. 2001;6:31–4. [PubMed] [Google Scholar]
- 36.Kuo HK, Yu YH. The relation of peripheral arterial disease to leg force, gait speed, and functional dependence among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:384–90. doi: 10.1093/gerona/63.4.384. [DOI] [PubMed] [Google Scholar]
- 37.Evans NS, Liu K, Criqui MH, et al. Associations of calf skeletal muscle characteristics and peripheral nerve function with self-perceived physical functioning and walking ability in persons with peripheral artery disease. Vasc Med. 2011;16:3–11. doi: 10.1177/1358863X10395656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–4. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 39.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM R. 2009;1:916–24. doi: 10.1016/j.pmrj.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150–6. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 41.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002;82:128–37. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- 42.Gardner AW, Womack CJ, Sieminski DJ, Montgomery PS, Killewich LA, Fonong T. Relationship between free-living daily physical activity and ambulatory measures in older claudicants. Angiology. 1998;49:327–37. doi: 10.1177/000331979804900501. [DOI] [PubMed] [Google Scholar]
- 43.Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med. 1997;2:286–91. doi: 10.1177/1358863X9700200402. [DOI] [PubMed] [Google Scholar]
- 44.Askew CD, Green S, Walker PJ, et al. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg. 2005;41:802–7. doi: 10.1016/j.jvs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 45.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDermott MM, Liu K, Carroll TJ, et al. Superficial femoral artery plaque and functional performance in peripheral arterial disease: walking and leg circulation study (WALCS III) JACC Cardiovasc Imaging. 2011;4:730–9. doi: 10.1016/j.jcmg.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–59. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]


