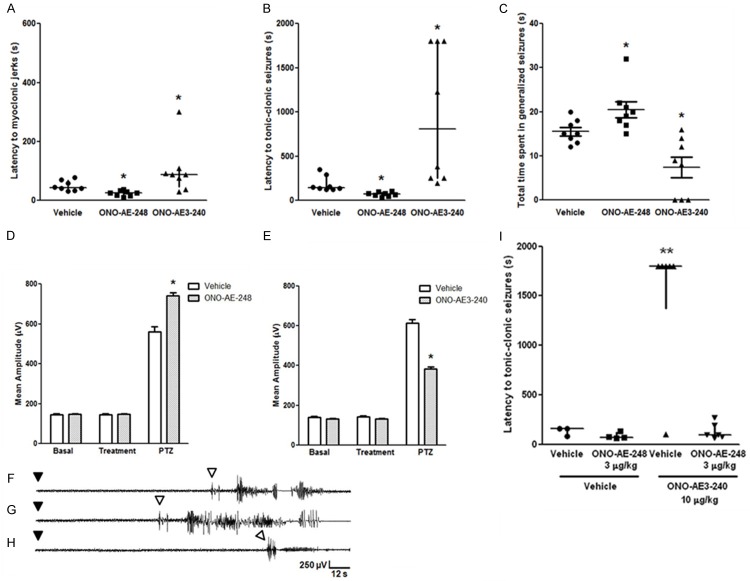
Figure 3.
(A-C) Effect of EP3 receptor agonist and antagonist (ONO-AE-248 and ONO-AE3-240, respectively; 10 µg/kg, s.c.) on PTZ-induced seizures (60 mg/kg, i.p.). (A) Latency to myoclonic jerk. (B) Latency to tonic-clonic generalized seizure. (C) Total time spent in generalized seizures. Data expressed as median and interquartile range (A and B), and mean + SEM (C), for n = 8 in each experimental group. (D, E) Effect of EP3 receptor agonist (D) and antagonist (E) (ONO-AE-248 and ONO-AE3-240, respectively; 10 µg/kg, s.c.) on the mean amplitude of EEG recordings in the parietal cortex of animal injected with PTZ (60 mg/kg, i.p.). Mean amplitude of EEG recordings was analyzed by two-way ANOVA followed by the Bonferroni’s test and expressed as mean + S.E.M., for n = 8 in each group. (F-H) Representative electrocorticographic recordings of animals after PTZ injection are represented as follows: (F) vehicle, (G) ONO-AE-248, and (H) ONO-AE3-240. Black and white arrowheads indicate PTZ injection and seizures latency, respectively, and the y-axis (amplitude) and x-axis (time) calibration bar is the same for all traces. (I) Effect of ONO-AE-248 (3 µg/kg, s.c.) administration, followed or not by ONO-AE3-240 (10 µg/kg, s.c.), on PTZ-induced seizures, measured as latency to the first tonic-clonic seizure (I). ONO-AE-248 (3 µg/kg, s.c.) prevented the protective effect of ONO-AE3-240 (10 µg/kg, s.c.) against PTZ-induced seizures. Data expressed as median and interquartile range for n = 3-6 in each group. A probability of P < 0.05 was considered significant. *P < 0.05, and **P < 0.01, when compared with vehicle or vehicle + vehicle group.