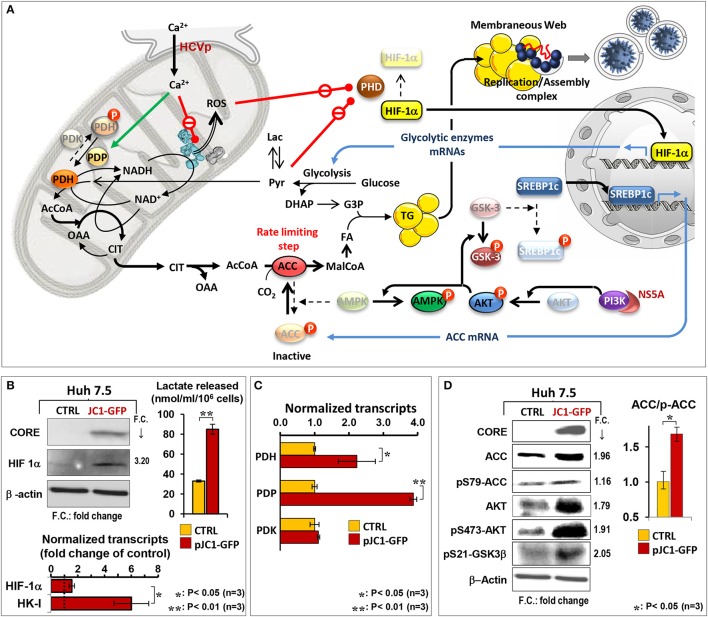
Figure 2.
HCV induces rewiring of cell metabolism in infected cells. (A) The scheme summarizes major changes in the metabolic pathways induced by HCV infection as supported by evidence reported in the literature and by the unpublished results showed in panels (B–D) obtained in HCV Jc1 RNA-transfected Huh-7.5 cells in vitro. HCV protein-induced enhanced entry of Ca2+ into mitochondria is shown to dampen the respiratory chain activity and oxidative phosphorylation (OxPhos) and to elicit increased reactive oxygen species (ROS) production. These inhibit the prolyl-hydroxylase (PHD) leading to stabilization of the hypoxia induced transcription factor (HIF-1α) which controls the expression of the glycolytic enzymes, thereby shifting cell metabolism toward aerobic glycolysis. (B) shows the stabilization of HIF-1α and the consequent metabolic shift evidenced by upregulation of the hexokinase I (HK-I) transcript and by increased lactate release in transfected Huh-7.5 cells. The enhanced glycolytic flux leads to accumulation of pyruvate which proved to further inhibit PHD. Pyruvate enters into mitochondria where it is converted in acetyl-CoA (AcCoA) by the pyruvate dehydrogenase (PDH). The HCV protein-mediated load of Ca2+ into mitochondria is shown to activate the pyruvate dehydrogenase phosphate (PDP), which controls the activity of the PDH. To note, at the transcriptional level both PDH and PDP [but not the pyruvate dehydrogenase kinase (PDK)] are significantly up-regulated in HCV RNA-transfected Huh-7.5 cells (C). The enhanced production of AcCoA leads to formation of citrate (CIT), which because of the limited availability of oxidized NAD+ (caused by impaired respiratory chain activity) is not further transformed via the tricarboxylic cycle and exits from mitochondria to shuttle AcCoA in the cytosol. The cytosolic AcCoA functions as precursor for the de novo synthesis of fatty acids (FA) that with intermediates of glycolysis forms triglycerides (TG) accumulating as lipid droplets. (D) shows that HCV RNA-transfected Huh-7.5 cells displays a two-fold increased expression of the acetyl CoA carboxylase (ACC), the controlling step in FA synthesis. ACC activity is controlled by its inactivating phosphorylation mediated by the AMP-activated protein kinase (AMPK) which is in turn controlled by the phosphorylated state of Akt/protein kinase B. Phosphorylation of AKT is mediated by activation of the phosphatidylinositol 3-kinase (PI3K), which has been reported to interact with HCV NS5A. Notably, the phosphorylated AKT is known to inactivate the glycogen synthase kinase 3β (GSK3β) which inhibits the activity of the transcription factor sterol regulatory element-binding protein 1c (SREBP 1c) controlling the expression of ACC. Consistently, the Western blots in panel (D) show enhanced phosphorylation of both AKT and GSK3β. The inability of HCV RNA-transfected to properly oxidize FA by the mitochondrial β-oxidation (requiring efficient respiratory chain) may lead to cytosolic accumulation of acyl-CoA which flow into TG synthesis (not shown). Lipid droplets results in formation of a membraneous web, contributed also by the HCV protein induced extensive rearrangement of host cell membranes, which contains the sites of viral replication and possibly assembly.