Fig. 2.
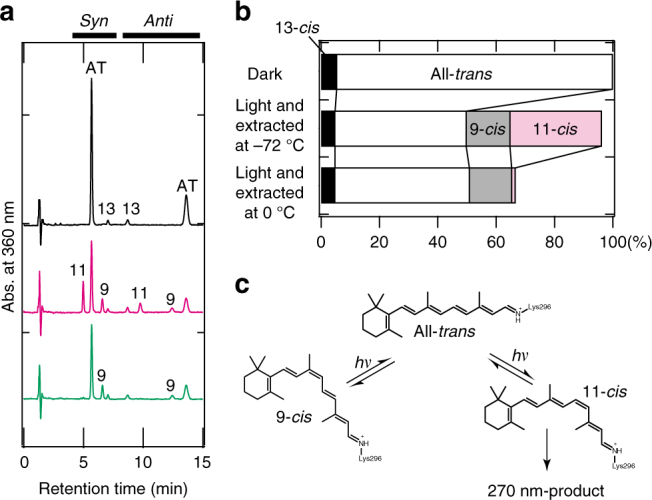
Retinal configurations in the intermediates produced by irradiation of Opn5L1 at −72 °C. a HPLC patterns of the retinaloximes extracted from non-irradiated Opn5L1NC sample (black), cooled at −72 °C in an ethanol/dry ice bath and irradiated with >500 nm light for 1 min (magenta), and subsequent incubation at 0 °C for 30 min (green). b Calculated compositions of retinal isomers in the samples based on each peak area in the chromatogram from a and the extinction coefficients previously reported55. Compositions of the retinal isomers of the dark sample (black in a) were 5.50 and 94.5% for the 13-cis and all-trans, respectively. Those of the sample after >500 nm light irradiation for 1 min and extraction at −72 °C (magenta in a) were 4.86, 45.0, 15.0, and 31.3% for the 13-cis, all-trans, 9-cis and 11-cis, respectively. Those of the sample after >500 nm light irradiation for 1 min at −72 °C, followed by extraction after incubation at 0 °C for 30 min (green in a) were 4.88, 46.1, 14.5, and 1.19% for the 13-cis, all-trans, 9-cis and 11-cis, respectively. c Reaction scheme of the chromophore of Opn5L1NC under irradiation with >500 nm light at −72 °C. It should be noted that only the 11-cis isomer was not extracted after incubation of the irradiated sample at 0 °C
