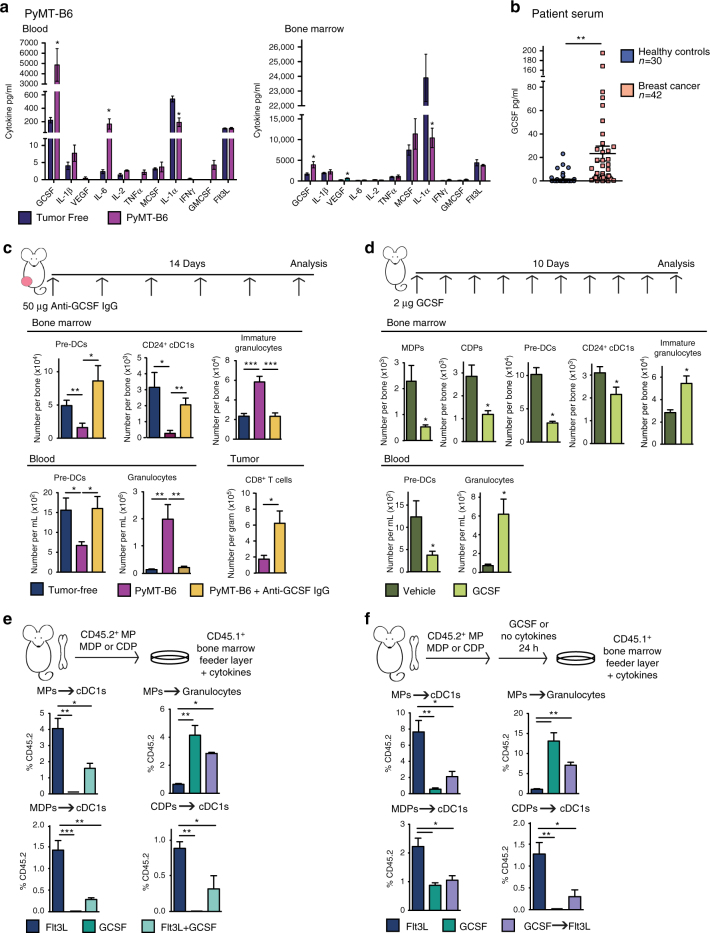
Fig. 4.
GCSF disrupts cDC1 differentiation. a Blood and BM serum cytokines in orthotopic PyMT-B6 end-stage tumor-bearing mice relative to tumor-free controls; tumor-free, n = 6; PyMT-B6, n = 7. b GCSF in human BC patient blood serum relative to healthy controls; healthy controls, n = 30; BC, n = 42. c Number of BM pre-DCs, CD24+ cDC1s, and immature granulocytes; numbers of blood pre-DCs and granulocytes, and tumor CD8+ T cells (CD45+CD3+CD8+) in tumor-free mice, orthotopic PyMT-B6 tumor-bearing end-stage mice, and orthotopic PyMT-B6 tumor-bearing end-stage mice treated for 2 weeks with 50 μg anti-GCSF IgGs 3 × /week; n = 5–7/group. Data are representative of two independent experiments. d Number of BM MDPs, CDPs, pre-DCs, CD24+ cDC1s, and immature granulocytes, and number of blood pre-DCs and granulocytes in C57BL/6 mice treated with 2 μg GCSF for 10 days; n = 6/group. Data are representative of two independent experiments. e CD45.2+Lin−Sca1−cKit+ MPs, MDPs, and CDPs isolated from tumor-free mice were cultured on CD45.1+ BM feeder culture in the presence of 100 ng/ml Flt3L, 100 ng/ml GCSF, or 100 ng/ml Flt3L and 100 ng/ml GCSF for 5 days. Final cultures were analyzed for cDC1s (Live CD45.2+CD45.1-MHCII+CD11c+Sirpα-CD24+) and granulocytes (Live CD45.2+CD45.1-CD11b+Ly6G+). Data are representative of three independent experiments consisting of three wells per condition. f Experiment similar to that in Fig. 4d, but CD45.2+ cells were pre-treated with 100 ng/ml GCSF or media alone for 24 h prior to plating on CD45.1+ BM feeder layer. Data are representative of two independent experiments consisting of three wells per condition. End stage for each model is defined in the Methods. Error bars represent mean+/− s.e.m. or box plot; *p < 0.05, **p < 0.01, ***p < 0.001 by unpaired two-sided Student’s t test