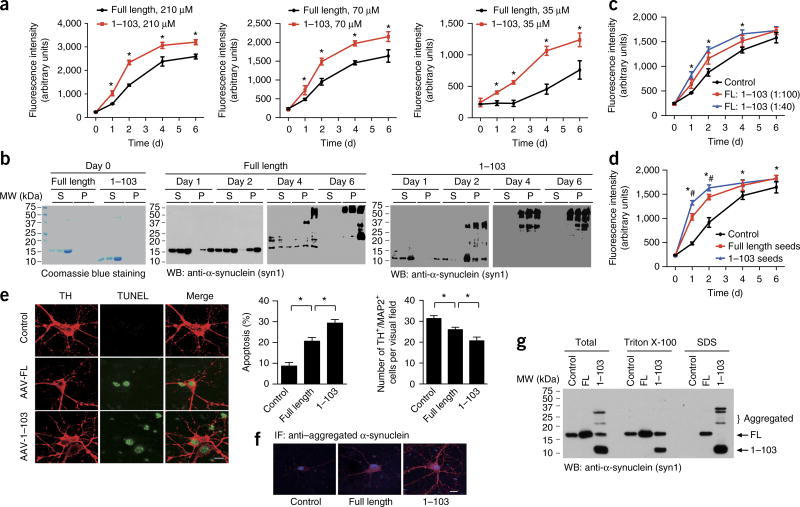
Figure 4.
AEP cleavage of α-synuclein promotes α-synuclein aggregation and toxic effects. (a) Thioflavin T assay showing the kinetics of aggregation of purified recombinant FL α-synuclein and the α-synuclein1–103 fragment at different concentrations. Data are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 by two-tailed Student’s t test. (b) Ultracentrifugation assay. The soluble and aggregated α-synuclein was analyzed by western blotting (S, supernatant; P, pellet). The blots shown are representative of three independent experiments. (c) The α-synuclein1–103 fragment promotes the aggregation of FL α-synuclein. 70 µM α-synuclein aggregation was assessed with thioflavin T assays in the presence of 1:100 or 1:40 α-synuclein1–103 fragment. Data are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 compared with control, by one-way ANOVA. (d) Seeding of full-length α-synuclein by fibrils of the α-synuclein1–103 fragment (blue) or fibrils of FL α-synuclein (red). Data are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 compared with control; #P < 0.05 compared with fibrils from FL α-synuclein, by one-way ANOVA. (e) The neurotoxicity of FL α-synuclein and the α-synuclein1–103 fragment. α-Synuclein (without tag) was expressed in primary cultured SN neurons. Cell death was determined by TUNEL staining and TH+/MAP2+ cell counts. Scale bar for micrographs, 20 µm. Data shown in graphs are mean ± s.e.m.; n = 3 independent experiments; *P < 0.05 by one-way ANOVA. (f) Immunostaining showing the aggregation of α-synuclein. Scale bar, 20 µm. (g) Western blot (WB) showing the aggregation of α-synuclein1–103 fragments into higher-molecular-weight species. Blots shown are representative of three independent experiments; uncropped images are shown in Supplementary Data Set 1. Source data for graphs are available online.