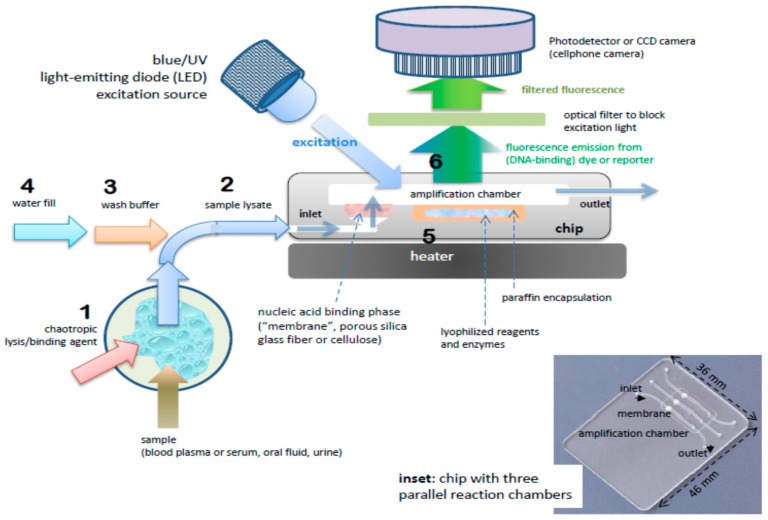
Figure 2.
Process steps and (cross-section) schematic of chip for nucleic acid amplification test (NAAT). Chip has one or more flow-through chambers for isothermal amplification and includes a filter-like, flow-porous nucleic acid binding phase (e.g., silica glass fiber or cellulose) and is pre-loaded with paraffin-encapsulated amplification reagents (lyophilized polymerase, primers, fluorescence reporter DNA-intercalating, dyes, and other components). Operational steps: (1) sample is mixed off-chip with lysis/binding reagent buffer (containing e.g., chaotropic agent such as guanidinium HCl) that lyses virus and cells and promotes nucleic acid adsorption to binding media, e.g., silica glass fiber or cellulose (‘membrane’), (2) sample (~100 µL) is injected into chip with pipette or syringe, (3) ethanol-based, high-salt buffer (~100 µL) is injected into chip to wash the membrane (keeping most of the captured nucleic acid adsorbed to the membrane, (4) chamber (25 to 50 µL volume) is filled with water and sealed with tape, (5) chip is heated to amplification temperature (~65 °C) using a small (~1 Watt) electric-heater. The heating melts the paraffin encapsulation, releasing and reconstituting the reagents, (6) the amplification reaction is excited with a blue or UV LED, such that the DNA intercalating dye generates a fluorescence signal proportional to the amount of DNA amplicon produced. The fluorescence is measured by filtering the excitation light and detection with a photodetector of CCD camera, such as provided by a mounted cellphone.