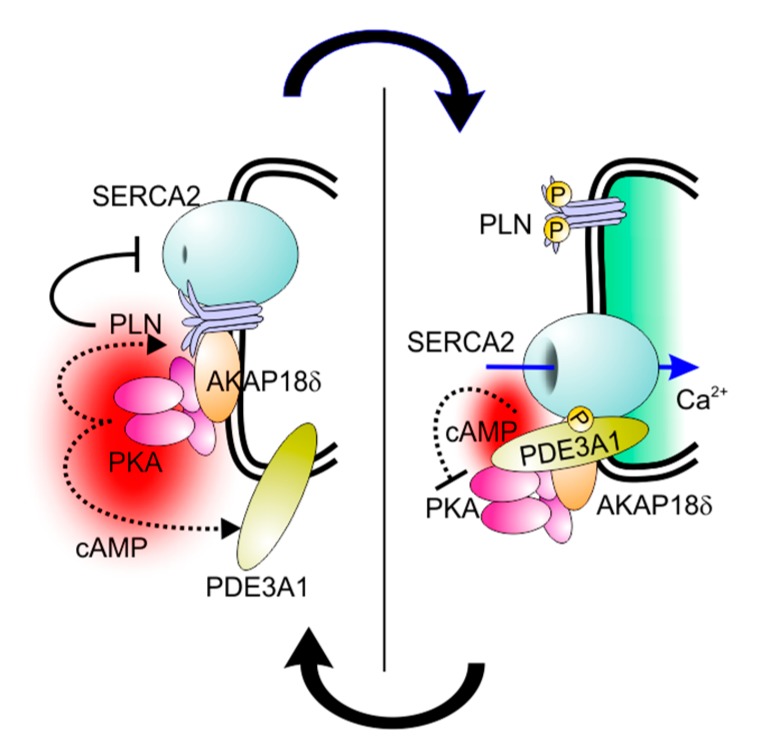
Figure 2.
Control of SERCA2 activity by PDE3A. Left panel: Under resting conditions, phospholamban (PLN) binds to SERCA2, whose activity it inhibits, as well as AKAP18δ on the cytoplasmic surface of the sarcoplasmic reticulum (double black line). Upon β-adrenergic receptor-stimulated cAMP production, PKA associated with AKAP18δ is activated and phosphorylates PLN (upper dotted line) and PDE3A1 (lower dotted line). This leads to the condition shown in the right panel, where phosphorylated PLN detaches from SERCA2 and loses its inhibitory action. As a consequence, SERCA2-dependent Ca2+ uptake into the lumen of the sarcoplasmic reticulum is stimulated (encircled by the double black line). At the same time, phosphorylated PDE3A1 binds to SERCA2 and increases its cAMP-hydrolytic activity, which limits the extent to which β-adrenergic receptor-mediated signalling amplifies intracellular Ca2+ cycling.