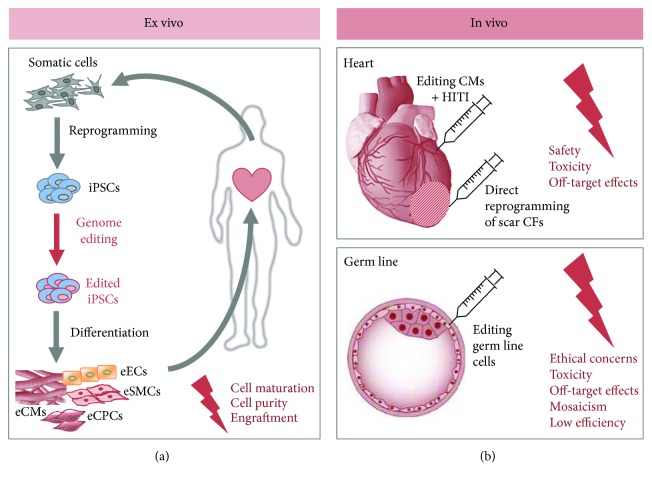
Figure 2.
Genome editing for regenerative medicine. The future application of genome editing techniques in vivo for regenerative therapies in the cardiovascular field is still in the early stages of development. This figure shows the potential and remaining challenges of genome editing for regenerative therapies. One option is to produce iPSCs from a patient and edit the gene of interest ex vivo (a). After editing the iPSCs, they can be expanded, differentiated into the desired cell type, and transplanted back into the patient. Remaining problems are mainly cell maturation and purification issues as well as low engraftment after transplantation. The other option is in vivo genome editing by directly targeting the gene of interest in the host organism. With the implementation of homology-independent targeted integration (HITI), precise genome editing is even possible in nondividing cells like cardiomyocytes (b). However, besides safety and toxicity issues, off-target effects have to be entirely excluded before clinical application. Many genetic diseases cannot be cured with targeting somatic cells, thereby demanding the use of germ-line editing. But genome editing in human embryos is of course highly controversial, so that safety and ethical concerns need to be fully addressed before moving on to clinical application. iPSCs: induced pluripotent stem cells; eECs: edited endothelial cells; eSMCs: edited smooth muscle cells; eCMs: edited cardiomyocytes; eCPCs: edited cardiac progenitor cells; CMs: cardiomyocytes; CFs: cardiac fibroblasts; HITI: homology-independent targeted integration.