Table 1. Optimization of reaction conditions a .
| ||||
| Entry | Reaction condition deviation | Vinylsilanes | Product | Yields of 15 (%) |
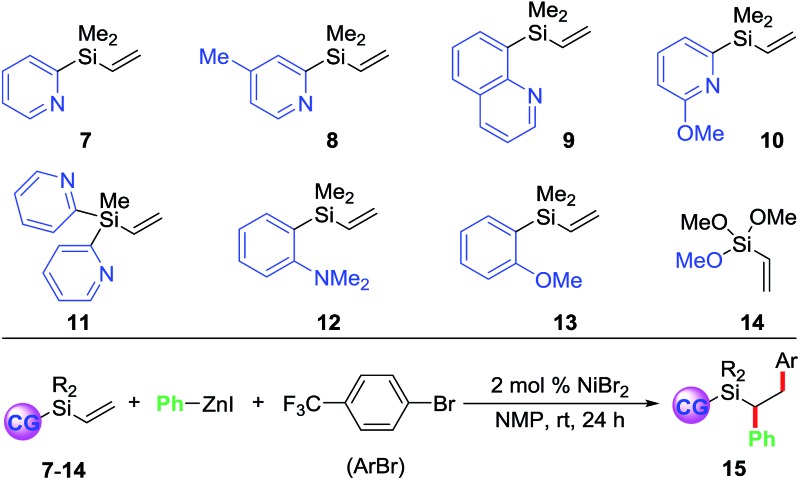
| 1 | None | 7 | 15a | 79 (72) |
| 2 | None | 8 | 15b | 51 |
| 3 | None | 9 | 15c | 56 |
| 4 | None | 10 | 15d | 0 |
| 5 | None | 11 | 15e | 0 |
| 6 | None | 12 | 15f | 0 |
| 7 | None | 13 | 15g | 0 |
| 8 | None | 14 | 15h | 0 |
| 9 | 15 h | 7 | 15a | 62 |
| 10 | (cod)2Ni instead of NiBr2 | 7 | 15a | 35 |
| 11 | (Ph3P)4Ni instead of NiBr2 | 7 | 15a | 30 |
| 12 | DMF or DMA instead of NMP | 7 | 15a | 40–44 |
| 13 | DMSO, dioxane or MeCN instead of NMP | 7 | 15a | 10–15 |
| 14 | Benzene of THF instead of NMP | 7 | 15a | <5 |
| 15 | Cul or FeCl3 instead of NiBr2 | 7 | 15a | 0 |
| 16 | Pd(OAc)2 or Co(OAc)2 instead of NiBr2 | 7 | 15a | 0 b |
aYields were determined by 1H NMR using pyrene as an internal standard. Value in parenthesis is the isolated yield from a 0.5 mmol scale reaction.
bHeck product was formed in >90% NMR yield with Co(OAc)2.
