Table 2. Oxidative halogenation of N-methyl sulfamate derivatives.
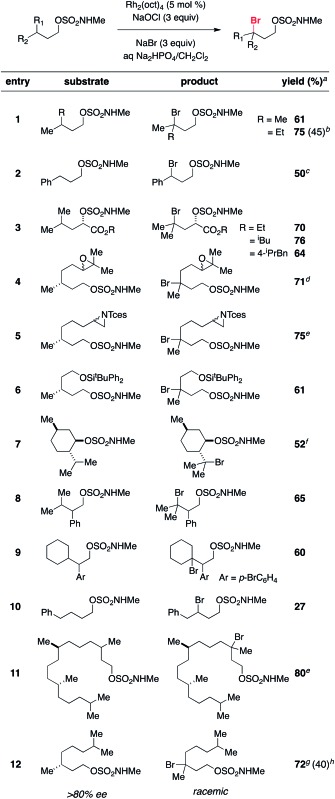
|
aIsolated product yield unless otherwise indicated.
bReaction performed with 0.1 mol% Rh2(oct)4.
cYield estimated by 1H NMR integration using an internal standard.
dProduct isolated as a 1 : 1 mixture of diastereomers.
eProduct isolated as a mixture of diastereomers, ratio undetermined.
fProduct yield estimated by 1H NMR integration using an internal standard. Chromatography on SiO2 facilitates bromide elimination, see Fig. S1 for details.
gProduct isolated as a racemic mixture, see Fig. S2 for details.
hYield of corresponding chloride product obtained from a reaction performed without NaBr.
