Figure 3.
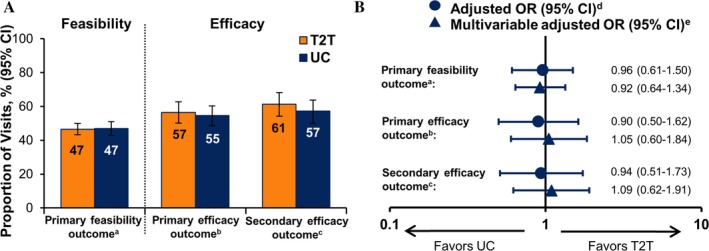
Outcomes in the treat‐to‐target (T2T) versus usual care (UC) groups at 12 months. A, Unadjusted response rates at 12 months. B, Odds ratio (OR) comparing response rates with T2T versus UC at 12 months, adjusted for clustering and patient baseline characteristics. 95% CI = 95% confidence interval; a = the primary feasibility outcome was the probability of treatment acceleration conditional on Clinical Disease Activity Index score >10; b = the primary efficacy outcome was overall achievement of low disease activity (LDA); c = the secondary efficacy outcome was achievement of LDA among patients who completed the study (analysis of those who completed); d = T2T versus UC adjusted for clustering by physician for the efficacy outcomes and for clustering by patient, physician, and practice site for the feasibility outcomes; e = adjusted for age, Hispanic ethnicity, Medicare insurance, rheumatoid factor seropositivity, disease duration, number of prior biologic agents, number of prior conventional synthetic disease‐modifying antirheumatic drugs, current biologic agent/small molecule use, and clustering.
