Figure 1.
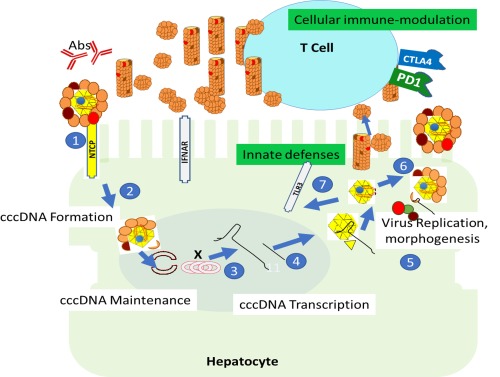
HBV life cycle, emphasizing opportunities to suppress viral cccDNA and restore immune control. Host and viral functions that could be exploited for therapeutic purposes are illustrated, beginning with binding of the virus to the sodium–taurocholate cotransporting polypeptide receptor on hepatocytes (1), followed by translocation of the nucleocapsid to the nucleus and formation of cccDNA (2) and synthesis steps (3,4), leading to either egress (6) of newly formed virions or recycling of cccDNA‐containing nucleocapsids to the nucleus (7). Opportunities for cccDNA suppression and immune control are categorized as either acting upon the viral gene products (white boxed text) or acting upon host innate and adaptive immune systems (green boxed text), noting that in many cases these different pathways overlap. Humoral responses are also indicated (Abs). Orange, red, and brown circles indicate small, medium, and large hepatitis B surface proteins, respectively; yellow triangle (core protein), blue circle (pol), “X” (x protein), red semicircles, cccDNA and black line, HBV 3.2 kb and subgenomic HBV RNA; 22‐nM in diameter spherical and rod‐shaped subviral envelope particles and infectious 42‐nM virions are also illustrated. The examples of virus life cycle steps and immune modulators are representative and not comprehensive. Toll‐like receptor 3 is shown because it is present in hepatocytes, but other toll‐like receptors may also be exploited therapeutically. Abbreviations: Abs, antibodies; CTLA4, cytotoxic T lymphocyte antigen 4; IFNAR, type 1 interferon receptor; NTCP, sodium–taurocholate cotransporting polypeptide; PD1, programmed death 1; TLR3, Toll‐like receptor 3.
