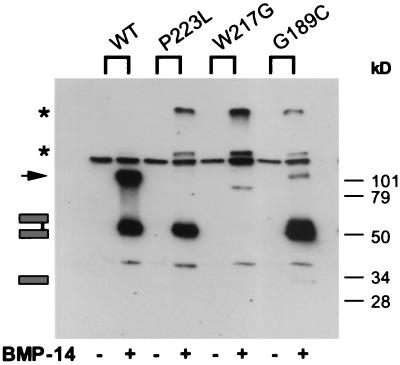
Figure 5.
Interaction of noggin mutant proteins and coexpressed BMP-14. Conditioned media from COS-7 cells cotransfected with pcDNA3 containing myc-tagged BMP-14 (+BMP-14) and pcDNA3 containing NOG wild-type sequence (WT), SYM1-derived sequences (P223L, G189C), or SYNS1-derived sequence (W217G). As negative controls, anti-myc immunoprecipitation was performed on the conditioned media from the noggin constructs expressed alone (−BMP-14). Samples were subjected to nonreducing SDS/8% PAGE and immunodetected using an anti-noggin antibody. Arrow denotes artifact band of noggin, and asterisks denote high molecular weight species of noggin. Band migrating between * and arrow is the anti-myc antibody. Wild-type noggin dimer and the two SYM1 mutant dimers coprecipitate with BMP14. Monomeric noggin does not coprecipitate with BMP-14. A much larger amount of dimeric noggin mutant (G189C) coprecipitates than might be expected based on this mutant's predominant secretion as a monomer when expressed alone (see Fig. 2).