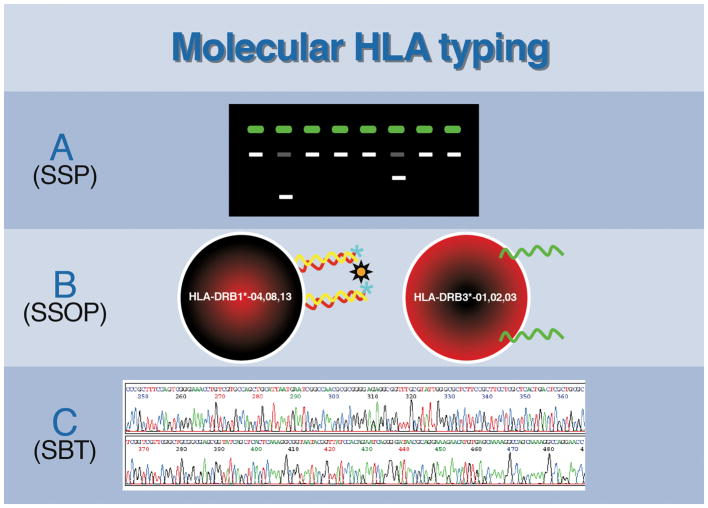
Figure 2.
In the beginning, the foundation of HLA testing was serologic in nature with an interdependence of antibodies to identify antigens, and antigens to identify antibodies. However, as time progressed, HLA laboratory testing took two distinct roads, one driven by the need for high resolution molecular characterization of HLA alleles, the other driven by the need to identify and classify HLA antibodies via serology. The directions these two paths took evolved from the clinical needs in stem cell and solid organ transplantation: Bone marrow/stem cell transplantation depends on accurate, reliable allele resolution to identify HLA-matched recipient-donor pairs while solid organ transplantation requires identification of HLA antibodies, specifically donor specific antibodies (DSA). Antigen testing led to high resolution HLA typing, which identifies individual HLA alleles; while antibody identification has progressed using solid-phase methods that now have the ability to identify allele-specific antibodies and characterize unique epitopes restricted to distinct HLA alleles. Eventually, the two roads of histocompatibility testing converged, with antigen and antibody testing coming full circle and once again each relying on the other. This convergence is exemplified in stem cell transplants, wherein donors expressing an HLA allele to which the recipient has a corresponding antibody must be considered before moving forward with a transplant. This requires high resolution antigen typing of the donor as well as high resolution antibody testing of the recipient to be performed. Similarly, since allele-specific HLA antibodies can be identified in solid organ transplant recipient, high resolution HLA typing of donors will be needed to determine compatibility.