Figure 2.
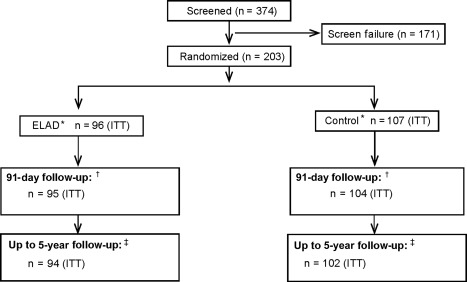
Flowchart and disposition of patients during the study until July 2015. Of the 374 subjects screened for the study, 171 did not meet inclusion criteria or presented with exclusion criteria, most frequently the inability to provide informed consent (n = 39), MELD score > 35 (n = 44), evidence of reduction in total bilirubin of 20% or more in the previous 72 hours (n = 42), and evidence of significant concomitant disease with expected life expectancy of <3 months (n = 29). *The ITT population is “as randomized.” Of the 96 subjects randomized to ELAD, 2 subjects deteriorated and became unstable before ELAD could be initiated; those subjects did not receive ELAD. One subject randomized to control received ELAD. In a separate safety analysis, the populations were analyzed “as treated.” †During the 91‐day follow‐up, 1 patient in the ELAD group and 1 in the control group were “lost to follow‐up” and 2 patients in the control group withdrew consent, so the outcome is known in 95 and 104 subjects in ELAD and control. ‡As of July 2015, using data from the VTI‐208 Extension study, the outcome is known for 94 ELAD and 102 Control subjects; 1 ELAD subject and 3 Control subjects were lost to follow‐up and 1 ELAD subject and 2 Control subjects withdrew consent.
