Abstract
PURPOSE
Primary aldosteronism (PA) is a common cause of secondary hypertension. Adrenal venous sampling (AVS) is the gold standard for assessing laterality of PA, which is of paramount importance to decide adequate treatment. AVS is a technically complicated procedure with success rates ranging between 30% and 96%. The aim of this study was to investigate the success rate of AVS over time, performed by a single interventionalist.
METHODS
This was a retrospective study based on consecutive AVS procedures performed by a single operator between September 2005 and June 2016. Data on serum concentrations of aldosterone and cortisol from right and left adrenal vein, inferior vena cava, and peripheral vein were collected and selectivity index (SI) calculated. Successful AVS was defined as SI >5.
RESULTS
In total, 282 AVS procedures were performed on 269 patients, 168 men (62%) and 101 women (38%), with a mean age of 55±11 years (range, 26–78 years). Out of 282 AVS procedures, 259 were successful, giving an overall success rate of 92%. The most common reason for failure was inability to localize the right adrenal vein (n=16; 76%). The success rates were 63%, 82%, and 94% during the first, second, and third years, respectively. During the last 8 years the success rate was 95%, and on average 27 procedures were performed annually.
CONCLUSION
Satisfactory AVS success rate was achieved after approximately 36 procedures and satisfactory success rate was maintained by performing approximately 27 procedures annually. AVS should be limited to few operators that perform sufficiently large number of procedures to achieve, and maintain, satisfactory AVS success rate.
Primary aldosteronism (PA) is the most common cause of secondary hypertension. The estimated prevalence of the disease is 5%–13% in hypertensive patients (1–3). Of several possible underlying causes of PA, the most common are unilateral aldosterone producing adrenal adenoma and bilateral idiopathic adrenal hyperplasia (4). In patients with confirmed PA, it is of paramount importance to determine whether the disease is unilateral or bilateral in order to decide appropriate treatment strategy. Unilateral PA can be successfully treated with adrenalectomy and bilateral disease with mineralocorticoid receptor antagonist (4).
Numerous methods, such as clinical characteristics, radiologic findings, the postural stimulation test, adrenal scintigraphy and adrenal vein sampling (AVS), have been used to assess laterality of PA. Except AVS, none has been shown to have acceptable sensitivity and specificity to evaluate if the aldosterone secretion is lateralized (5–7). The disadvantage of the AVS is the complexity of the procedure, resulting in limited availability. Indeed, AVS demands an experienced and dedicated interventionalist in order to achieve a satisfactory success rate (8). In previous reports the success rates varied greatly, from 30% up to 96% (8–10).
Except for one small study by Siracuse et al. (11), the learning curve of AVS has not been reported before. The study included 53 procedures performed by a single vascular surgeon during 6 years and showed an increasing success rate from 58% during the earlier years (2007–2010) compared with 82% after that (2011–2012) (11). The purpose of this study was to evaluate the success rate of AVS over time, performed by a single interventional radiologist, in a large cohort of patients with PA.
Methods
Study design
This was a retrospective single-center study based on data from consecutive AVS performed by a single operator between September 2005 and June 2016. Our department is the tertiary referral center for a region with a population of 1.7 million inhabitants.
Patients
Patients who had been investigated with AVS at our hospital from September 2005 to June 2016 were identified through search in the electronic medical records, and the registration system at the department of radiology.
All patients had initially been screened for PA by measuring aldosterone to renin ratio. A total of 232 patients (86%) had the diagnosis confirmed with an intravenous saline load test. Information on confirmatory test was missing in 27 patients (10%) and was not performed in 10 (4%).
Patient charts for all patients who underwent AVS during the study period were reviewed and the following data were collected and recorded: age, gender, body mass index, duration of hypertension, blood pressure, number and doses of antihypertensive medications, potassium supplementation, aldosterone to renin ratio at screening and at confirmatory testing.
Adrenal vein sampling
Since September 2005 all AVS procedures at our department were performed by a single interventional radiologist who had 7 years of experience in interventional radiology. The first two AVS procedures (excluded from this analysis) were performed under the guidance of an experienced Swedish interventional radiologist who performed 10–15 AVS procedures annually at that time.
Adrenocorticotropic hormone (ACTH) stimulation was used during all the procedures: 750 μg of ACTH (Synacthen; CD Pharma AB) was diluted in 800 mL of 0.9% saline, which was infused at a rate of 100 mL/h (94 μg/h), starting two hours prior to the procedure. The administration of ACTH during the procedures and the collection and management of all blood samples were taken care of by the same nurse.
In all cases the right femoral vein was punctured first and a SIM 1 (4 F) (Cordis), Cobra (4–5 F; Cordis), or Shepherd Hook (4–5 F; Angiodynamics) catheters were used to localize and draw blood from the right adrenal vein. Next, a SIM 2 (Cordis), or SIM 3 (4 F; Cordis) catheter was used to localize and draw blood from the left adrenal vein. Usually, one or two samples were drawn from left adrenal vein. These were drawn either at the junction of inferior phrenic and adrenal vein or selectively from left adrenal vein, few mm above the junction (cranially). Thereafter, a blood sample from the inferior vena cava was collected at a level below the right renal vein. Then the right adrenal vein was cannulated again for collection of a second blood sample. Finally, a blood sample from a peripheral vein was drawn. All catheters had one or more pairs of side holes, punched during the procedure, approximately 1–2 mm from the tip of the catheters. 5 F vascular sheath (Cordis) was always used. In several cases, 7 F renal double curve guiding catheter (Cordis) was introduced. Standard 0.035-inch guidewire (Starter, Boston Scientific) was used in all cases and 0.035-inch guidewire (Radiofocus, Terumo) was needed in a few cases. Microcatheter (Progreat, Terumo) was used in 3 procedures. Samples were drawn with 5 mL syringes.
In complicated cases, mainly when it was difficult to localize the right adrenal vein, small amounts of contrast were injected at locations where the adrenal vein was expected to be found. If a vein suspected to be an adrenal vein was identified, especially if it had a high-flow rate (characteristic for the adrenal veins), a blood sample was drawn. In these cases, as well as when the catheter tip was not clearly and selectively inside an adrenal vein, the blood sample was analyzed using a rapid cortisol assay. In case of a complicated AVS, the procedure was stopped when either 200 mL of intravenous contrast (300 mg/I/mL) had been used, or more than 60 min of fluoroscopy time was reached, whichever came first.
Before the AVS, all patients were investigated with computed tomography (CT) of the adrenal glands, slice thickness 3 mm or less, both unenhanced and contrast-enhanced. On the day of AVS, the images were reviewed by the interventionalist aiming to recognize the adrenal veins, with main focus on the right adrenal vein. Initially this was only done sporadically but has been a routine procedure since 2009.
Selectivity index (SI) was used to assess the adequacy of the adrenal vein cannulations and was calculated according to the formula:
Successful AVS was defined as SI >5, bilaterally.
In cases where multiple samples were collected from the same adrenal vein, the one with the highest cortisol concentration, and the corresponding aldosterone, were used for calculation of the lateralization index (LI) according to the formula:
Biochemical analyses
During the study period, serum aldosterone was measured with three radioimmunoassays. Between 2005 and October 2008, Adaltis MAIA was used with a coefficient of variation (CV) of 11%–14%. Between November 2008 and October 2014, Siemens Coat-A-Count was used (CV, 6%–10%). Since November 2014 to the end of the study, DiaSorin Liaison (CV, 8%–13%) was used.
Between 2005 and 2008, serum cortisol was measured with Bayer Centaur (CV, 5%–6.5%), between 2008 and 2011 with Roche Modular E (CV, 5%–7%), between 2011 and 2015 with Roche Cobas, Cortisol 1 (CV, 3%–4%) and since 2015 with Roche Cobas, Cortisol-II (CV, 2%–3%).
Ethical considerations
The study was approved by the Ethics Committee of our University and conducted according to the Declaration of Helsinki. All participants gave their informed consent prior to the procedures.
Statistical analysis
The statistical analyses were performed with SPSS, version 22.0 for Windows. Descriptive statistics for normally distributed data are presented as mean ± standard deviation (SD) and non-normally distributed data as median and interquartile range (IQR).
Results
From September 2005 to June 2016, 282 AVS procedures were performed in 269 patients with confirmed PA, 168 (62%) men and 101 (38%) women. The mean age at the AVS was 55±11 years (range, 26–78 years). At the time of diagnosis of PA the mean systolic blood pressure was 151±19 mmHg and mean diastolic blood pressure 89±11 mmHg. The median number of antihypertensive medications was 2 (IQR, 1–3). Sixteen (6%) patients were not receiving any antihypertensive medications, 57 patients were taking one (21%), 97 patients two (36%), 46 patients three (17%), and 22 patients four or more (8%). Of 230 patients (data missing in n=39), 159 (69%) had substitution with potassium chloride with a median daily dose of 5.3 g (IQR, 3–7.5 g).
In total, 259 of 282 AVS procedures were successful, giving an overall success rate of 92%. The reasons for unsuccessful AVS procedures (n=23) were right adrenal vein not identified (n=16), impossible to achieve a stable catheter position due to breathing (n=2), impossibility to aspirate blood sample from adrenal vein due to suction of the catheter to the vein wall (n=2), and left adrenal vein not identified (n=1). In addition, two AVS procedures were unsuccessful due to laboratory error (blood samples not diluted).
The rate of technically successful AVS was 63% during the first year and 82% during the second year (Table). Eight procedures were performed in the first year and 28 procedures were performed in the second year. Thereafter the success rate increased and varied between 82% and 100% and 18–36 AVS procedures were performed annually. During the last five years the mean success rate was 96%.
Table.
Number of technically successful and unsuccessful AVS between September 2005 and June 2016
| Year | Number of AVS | Technically successful AVS, n | Technically unsuccessful AVS, n | Technical success rate, (%) |
|---|---|---|---|---|
| 2005 | 8 | 5 | 3 | 63 |
| 2006 | 28 | 23 | 5 | 82 |
| 2007 | 16 | 15 | 1 | 94 |
| 2008 | 26 | 24 | 2 | 92 |
| 2009 | 18 | 15 | 3* | 83 |
| 2010 | 23 | 23 | - | 100 |
| 2011 | 23 | 19 | 4* | 83 |
| 2012 | 24 | 23 | 1 | 96 |
| 2013 | 31 | 30 | 1 | 97 |
| 2014 | 36 | 34 | 2 | 94 |
| 2015 | 33 | 32 | 1 | 97 |
| 2016 | 16 | 16 | - | 100 |
| Total | 282 | 259 | 23 | 92 |
AVS, adrenal venous sampling.
One AVS in 2009 and one in 2011 were unsuccessful due to laboratory error (blood samples not diluted).
Nine patients with technically unsuccessful AVS underwent a second procedure, of which eight were successful (89%). In fact, one of these patients underwent three AVS procedures, the first one was unsuccessful due to laboratory error and the right adrenal vein was not identified during the second one, while the third one was successful. Four additional patients underwent a second AVS due to other reasons: two due to inconclusive LI (LI between 3 and 4), one due to mismatch between aldosterone/cortisol ratio between the first and second right adrenal vein samples, and one patient who relapsed in PA after an initially successful resection of aldosterone producing adrenal adenoma. All these four procedures were successful.
The median SI in technically successful AVS was 29 (IQR, 22–39; range, 5–97) on the right side and 21 (IQR, 15–26; range, 6.4–78) on the left side. Of 16 technically unsuccessful AVS procedures where blood samples were collected, 14 (88%) had SI <2.
In 249 out of 282 AVS procedures (88%), two blood samples were drawn from the right adrenal vein. Of these, 229 (92%) were successful during both samplings and 14 (6%) were unsuccessful on both occasions. Eight samplings (3%) were successful only during the second sampling and one (<1%) only during the first sampling.
No serious complications occurred in connection with AVS. Two patients complained of vague abdominal discomfort after the procedure where extravascular deposition of contrast material had occurred. Subsequently, hematoma was ruled out on unenhanced upper abdominal CT in both cases. One patient developed pseudoaneurysm due to an accidentally punctured arterial branch from the femoral artery that was treated conservatively.
Discussion
This study is to our knowledge the largest study to date to have evaluated the learning curve of AVS. We demonstrate that satisfactory AVS success rate was achieved after two years, during which 36 procedures were performed. The success rate increased from 63% and 82% during the first two years to be more than 96% during the last five years.
The learning curve of AVS has been investigated only once before in a study by Siracuse et al. (11). In that study, AVS in 53 patients with PA, performed by a single vascular surgeon during six years (2007–2012), was analyzed. The authors demonstrated a success rate of 58% during the first four years (37 patients), which later increased to 82% during the last 3 years (16 patients) (11). Although the study included a significantly smaller number of patients, and had a lower success rate, the results are in line with the results of the current study.
Between June 1998 and September 2005, 16 AVS procedures were performed at our hospital by several interventional radiologists. Of these, only 6 were successful, giving a success rate of only 38%, which led to a change in the organization as well as routines in relation to the procedure. Thus, the results from the current report emphasize the importance of sufficient volume of AVS assigned to the interventionalist, as to achieve satisfactory successful rates, as well as to guarantee the maintenance of high rates.
The success rate in our study is among the highest reported (12). In some series a success rate as low as 30% has been reported (9). The success rate in the study by Siracuse et al. (11) was somewhat lower than in our cohort. This difference is probably explained by a larger number of patients that were studied at our hospital, on average 26 procedures annually, compared to 8 in the study by Siracuse et al. (11), again illustrating the importance of sufficiently high number of procedures performed annually. This is further supported by reports suggesting that limiting the procedures to few operators increases the success rate (8–10). Based on previous and current results, it is suggested that in centers conducting fewer than 20 procedures per year, AVS should be performed by a single interventionalist.
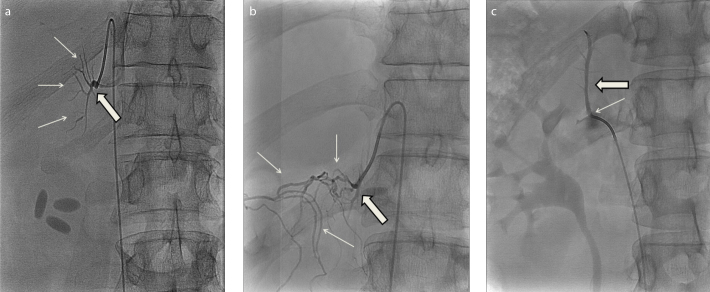
Universal instructions to a successful AVS are difficult to define. Without any doubt, important factors are experience, patience, and meticulousness of a dedicated interventionalist. There are, however, some supportive measures that can add to the technical success rate of AVS. One is the rapid serum cortisol analysis that can give a rapid feedback concerning the localization of the adrenal veins during the procedure (13, 14). Indeed, the success rate in our cohort was increased by 3% by collecting a second sample from the right adrenal vein. Rapid cortisol assay was most frequently used as a supportive tool at our hospital during the first three years of the current report and thereafter gradually declined, in parallel with increasing experience. Another measure is to study the anatomy of the right adrenal vein by using CT before the AVS. According to international guidelines (1), CT is recommended to be performed in all patients with confirmed PA in order to rule out malignancy, an exceptionally rare cause of PA (4). The main utility of CT in the clinical practice is, however, to provide the AVS operator with anatomical information (1). Previously it has been shown that by using thin-slice CT the right adrenal vein can be identified in 70%–95% of cases (15, 16). Unquestionably, review of the adrenal CT is of great importance in a substantial number of cases, especially when uncommon anatomical variants of the venous system are encountered (Fig.).
Figure. a–c.
Radiographic images from adrenal catheterizations showing normal anatomy of the right adrenal vein (a, thick arrow) draining with a sharp angle into the cava inferior approximately 2 cm above the orifice of the left renal vein, as well as small branches from the adrenal vein (a, thin arrows); anatomical variation of the right adrenal vein (b, thick arrow) with several collateral veins of unknown origin (b, thin arrows); and a rare anatomical variation in which the right adrenal vein (c, thick arrow) drains into the right renal vein near its orifice into the vena cava (c, thin arrow).
The important role of CT reference is further supported by a recent study by Morita et al. (17) where the success rate of inexperienced interventionalists was investigated. Three residents in diagnostic radiology performed in total 102 AVS procedures under supervision of experts and had a total success rate of 96% (SI >5). The good results were explained by guidance by an experienced interventionalist, who also assisted in demanding cases, but also by thorough studies of the anatomy of the adrenal veins on CT imaging prior to the procedure (17).
AVS has numerous potential sources of errors other than cannulation successfulness per se. Of major importance is the uniform management of the blood samples collected during the AVS, as in our study, all performed by the same nurse throughout the whole study period. Even how rapidly the blood samples are drawn from the adrenal veins can affect the hormone concentrations (lower if the sample is drawn rapidly). Furthermore, all our AVS procedures were performed using sequential sampling and not simultaneous sampling, i.e., when blood from the right and left adrenal veins are sampled at the same time (18). Although some authors suggest that the simultaneous method is more accurate, most experts consider a sequential sampling, performed under ACTH-stimulation, to be more reliable (19–21).
The major strengths of this study are the use of the same AVS protocol throughout the whole study period, conducted by the same interventionalist and the same nurse. The large number of patients is a major strength as well. There are, however, some limitations to the study, including its retrospective design. Also, the fact that all procedures were performed by the same interventionalist may jeopardize the generalizability of the study.
In conclusion, acceptable AVS success rate is possible to achieve after approximately 36 procedures, performed by a single interventionalist. The study stresses the importance of limiting AVS to few operators who perform at least 20 procedures annually in order to achieve, as well as to maintain, a high AVS success rate.
Main points.
Adrenal venous sampling (AVS), the gold standard for assessing laterality of primary aldosteronism, is a technically complicated procedure with success rates ranging between 30% and 96%.
In this retrospective study we have analyzed consecutive AVS procedures performed by a single operator during 12 years.
Out of 282 AVS procedures, 259 were successful, giving an overall success rate of 92%.
The success rate increased from 63% to 82% during the first two years, and it was stabilized at around 95% thereafter.
AVS should be limited to few operators that perform sufficiently large number of procedures to achieve, and maintain, satisfactory AVS success rate.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: An Endocrine Society clinical practice guideline. J Clin endocrinol Metabol. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. https://doi.org/10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 2.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. https://doi.org/10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Hannemann A, Bidlingmaier M, Friedrich N, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167:7–15. doi: 10.1530/EJE-11-1013. https://doi.org/10.1530/EJE-11-1013. [DOI] [PubMed] [Google Scholar]
- 4.Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) 2007;66:607–618. doi: 10.1111/j.1365-2265.2007.02775.x. https://doi.org/10.1111/j.1365-2265.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 5.Mulatero P, Bertello C, Rossato D, et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab. 2008;93:1366–1371. doi: 10.1210/jc.2007-2055. https://doi.org/10.1210/jc.2007-2055. [DOI] [PubMed] [Google Scholar]
- 6.Lau JH, Sze WC, Reznek RH, et al. A prospective evaluation of postural stimulation testing, computed tomography and adrenal vein sampling in the differential diagnosis of primary aldosteronism. Clin Endocrinol (Oxf) 2012;76:182–188. doi: 10.1111/j.1365-2265.2011.04202.x. https://doi.org/10.1111/j.1365-2265.2011.04202.x. [DOI] [PubMed] [Google Scholar]
- 7.Nomura K, Kusakabe K, Maki M, Ito Y, Aiba M, Demura H. Iodomethylnorcholesterol uptake in an aldosteronoma shown by dexamethasone-suppression scintigraphy - relationship to adenoma size and functional-activity. J Clin Endocr Metab. 1990;71:825–830. doi: 10.1210/jcem-71-4-825. https://doi.org/10.1210/jcem-71-4-825. [DOI] [PubMed] [Google Scholar]
- 8.Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf) 2009;70:14–17. doi: 10.1111/j.1365-2265.2008.03450.x. https://doi.org/10.1111/j.1365-2265.2008.03450.x. [DOI] [PubMed] [Google Scholar]
- 9.Vonend O, Ockenfels N, Gao X, et al. Adrenal venous sampling: evaluation of the German Conn’s registry. Hypertension. 2011;57:990–995. doi: 10.1161/HYPERTENSIONAHA.110.168484. https://doi.org/10.1161/HYPERTENSIONAHA.110.168484. [DOI] [PubMed] [Google Scholar]
- 10.Harvey A, Kline G, Pasieka JL. Adrenal venous sampling in primary hyperaldosteronism: Comparison of radiographic with biochemical success and the clinical decision-making with “less than ideal” testing. Surgery. 2006;140:847–853. doi: 10.1016/j.surg.2006.07.026. https://doi.org/10.1016/j.surg.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Siracuse JJ, Gill HL, Epelboym I, et al. The vascular surgeon’s experience with adrenal venous sampling for the diagnosis of primary hyperaldosteronism. Ann Vasc Surg. 2014;28:1266–1270. doi: 10.1016/j.avsg.2013.10.009. https://doi.org/10.1016/j.avsg.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–1233. doi: 10.1016/j.surg.2004.06.051. https://doi.org/10.1016/j.surg.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Reardon MA, Angle JF, Abi-Jaoudeh N, et al. Intraprocedural cortisol levels in the evaluation of proper catheter placement in adrenal venous sampling. J Vasc Interv Radiol. 2011;22:1575–1580. doi: 10.1016/j.jvir.2011.05.005. https://doi.org/10.1016/j.jvir.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Auchus RJ, Michaelis C, Wians FH, Jr, et al. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann Surg. 2009;249:318–321. doi: 10.1097/SLA.0b013e3181961d77. https://doi.org/10.1097/SLA.0b013e3181961d77. [DOI] [PubMed] [Google Scholar]
- 15.Morita S, Nishina Y, Yamazaki H, Sonoyama Y, Ichihara A, Sakai S. Dual adrenal venous phase contrast-enhanced MDCT for visualization of right adrenal veins in patients with primary aldosteronism. Eur Radiol. 2016;26:2073–2077. doi: 10.1007/s00330-015-4073-9. https://doi.org/10.1007/s00330-015-4073-9. [DOI] [PubMed] [Google Scholar]
- 16.Degenhart C, Strube H, Betz MJ, et al. CT mapping of the vertebral level of right adrenal vein. Diagn Interv Radiol. 2015;21:60–66. doi: 10.5152/dir.2014.14026. https://doi.org/10.5152/dir.2014.14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita S, Yamazaki H, Sonoyama Y, Nishina Y, Ichihara A, Sakai S. Successful adrenal venous sampling by non-experts with reference to CT images. Cardiovasc Intervent Radiol. 2016;39:1001–1006. doi: 10.1007/s00270-016-1335-0. https://doi.org/10.1007/s00270-016-1335-0. [DOI] [PubMed] [Google Scholar]
- 18.Schirpenbach C, Seiler L, Maser-Gluth C, Beuschlein F, Reincke M, Bidlingmaier M. Automated chemiluminescence-immunoassay for aldosterone during dynamic testing: comparison to radioimmunoassays with and without extraction steps. Clin Chem. 2006;52:1749–1755. doi: 10.1373/clinchem.2006.068502. https://doi.org/10.1373/clinchem.2006.068502. [DOI] [PubMed] [Google Scholar]
- 19.Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63:151–160. doi: 10.1161/HYPERTENSIONAHA.113.02097. https://doi.org/10.1161/HYPERTENSIONAHA.113.02097. [DOI] [PubMed] [Google Scholar]
- 20.Carr CE, Cope C, Cohen DL, Fraker DL, Trerotola SO. Comparison of sequential versus simultaneous methods of adrenal venous sampling. J Vasc Interv Radiol. 2004;15:1245–1250. doi: 10.1097/01.RVI.0000134495.26900.6A. https://doi.org/10.1097/01.RVI.0000134495.26900.6A. [DOI] [PubMed] [Google Scholar]
- 21.Monticone S, Satoh F, Giacchetti G, et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension. 2012;59:840–846. doi: 10.1161/HYPERTENSIONAHA.111.189548. https://doi.org/10.1161/HYPERTENSIONAHA.111.189548. [DOI] [PubMed] [Google Scholar]